Hemoglobin S and C affect biomechanical membrane properties of P. falciparum-infected erythrocytes
- PMID: 31428699
- PMCID: PMC6692299
- DOI: 10.1038/s42003-019-0556-6
Hemoglobin S and C affect biomechanical membrane properties of P. falciparum-infected erythrocytes
Abstract
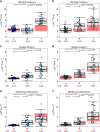
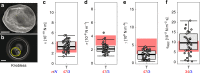
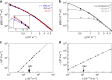
During intraerythrocytic development, the human malaria parasite Plasmodium falciparum alters the mechanical deformability of its host cell. The underpinning biological processes involve gain in parasite mass, changes in the membrane protein compositions, reorganization of the cytoskeletons and its coupling to the plasma membrane, and formation of membrane protrusions, termed knobs. The hemoglobinopathies S and C are known to partially protect carriers from severe malaria, possibly through additional changes in the erythrocyte biomechanics, but a detailed quantification of cell mechanics is still missing. Here, we combined flicker spectroscopy and a mathematical model and demonstrated that knob formation strongly suppresses membrane fluctuations by increasing membrane-cytoskeleton coupling. We found that the confinement increased with hemoglobin S but decreases with hemoglobin C in spite of comparable knob densities and diameters. We further found that the membrane bending modulus strongly depends on the hemoglobinopathetic variant, suggesting increased amounts of irreversibly oxidized hemichromes bound to membranes.
Keywords: Membrane biophysics; Parasitic infection.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures




References
-
- Fung, Y., Skalak, R. Biomechanics: mechanical properties of living tissues. (Springer Science & Business Media, Berlin, Germany, 2013).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

