The autoregulator Aca2 mediates anti-CRISPR repression
- PMID: 31428783
- PMCID: PMC6765145
- DOI: 10.1093/nar/gkz721
The autoregulator Aca2 mediates anti-CRISPR repression
Abstract
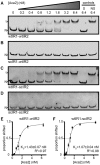
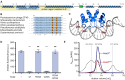
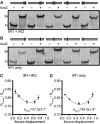
CRISPR-Cas systems are widespread bacterial adaptive defence mechanisms that provide protection against bacteriophages. In response, phages have evolved anti-CRISPR proteins that inactivate CRISPR-Cas systems of their hosts, enabling successful infection. Anti-CRISPR genes are frequently found in operons with genes encoding putative transcriptional regulators. The role, if any, of these anti-CRISPR-associated (aca) genes in anti-CRISPR regulation is unclear. Here, we show that Aca2, encoded by the Pectobacterium carotovorum temperate phage ZF40, is an autoregulator that represses the anti-CRISPR-aca2 operon. Aca2 is a helix-turn-helix domain protein that forms a homodimer and interacts with two inverted repeats in the anti-CRISPR promoter. The inverted repeats are similar in sequence but differ in their Aca2 affinity, and we propose that they have evolved to fine-tune, and downregulate, anti-CRISPR production at different stages of the phage life cycle. Specific, high-affinity binding of Aca2 to the first inverted repeat blocks the promoter and induces DNA bending. The second inverted repeat only contributes to repression at high Aca2 concentrations in vivo, and no DNA binding was detectable in vitro. Our investigation reveals the mechanism by which an Aca protein regulates expression of its associated anti-CRISPR.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Dy R.L., Richter C., Salmond G.P.C., Fineran P.C.. Remarkable mechanisms in microbes to resist phage infections. Annu. Rev. Virol. 2014; 1:307–331. - PubMed
-
- Barrangou R., Fremaux C., Deveau H., Richards M., Boyaval P., Moineau S., Romero D.A., Horvath P.. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007; 315:1709–1712. - PubMed
-
- Jackson S.A., McKenzie R.E., Fagerlund R.D., Kieper S.N., Fineran P.C., Brouns S.J.J.. CRISPR-Cas: adapting to change. Science. 2017; 356:eaal5056. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

