The importance of 12R-lipoxygenase and transglutaminase activities in the hydration-dependent ex vivo maturation of corneocyte envelopes
- PMID: 31429091
- PMCID: PMC6899781
- DOI: 10.1111/ics.12574
The importance of 12R-lipoxygenase and transglutaminase activities in the hydration-dependent ex vivo maturation of corneocyte envelopes
Abstract
Background: Terminally differentiated keratinocytes acquire corneocyte protein envelopes (CPE) complexed with corneocyte lipid envelopes (CLE). These two structural components of the corneocyte envelopes (CEs) undergo maturation by gaining in hydrophobicity, rigidity and surface area. Linoleoyl acylceramides are processed by 12R-lipoxygenase (12R-LOX) and other enzymes before transglutaminase (TG) attaches ω-hydroxyceramides to involucrin in the CPE. Concurrently, structural proteins are cross-linked by TG that has been activated by cathepsin D (CathD).
Objectives: The primary aim of this work was to demonstrate the impact of relative humidity (RH) during ex vivo CE maturation. Low, optimal and high RH were selected to investigate the effect of protease inhibitors (PIs) on CE maturation and TG activity; in addition, 12R-LOX and CathD activity were measured at optimal RH. Finally, the effect of glycerol on ex vivo CE maturation was tested at low, optimal and high RH.
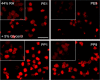
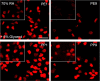
Methods: The first and ninth tape strip of photo-exposed (PE) cheek and photo-protected (PP) post-auricular sites of healthy volunteers were selected. Ex vivo CE maturation was assessed via the relative CE maturity (RCEM) approach based on CE rigidity and hydrophobicity. The second and eighth tapes were exposed to RH in the presence of inhibitors.
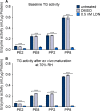
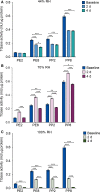
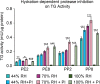
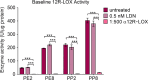
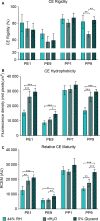
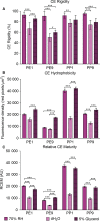
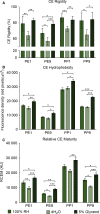
Results: Irrespective of tape stripping depth, CEs from PE samples attained CE rigidity to the same extent as mature CEs from the PP site, but such improvement was lacking for CE hydrophobicity. 70% RH was optimal for ex vivo CE maturation. The inhibition of 12R-LOX activity resulted in enhanced CE rigidity which was reduced by the TG inhibitor. CE hydrophobicity remained unchanged during ex vivo maturation in the presence of TG or 12R-LOX inhibition. CE hydrophobicity was enhanced in the presence of glycerol at 44% RH and 100% RH but not at 70% RH. Furthermore, TG activity was significantly diminished at 100% RH compared to the commercial inhibitor LDN-27219. However, a protease inhibitor mix reversed the negative effect of overhydration.
Conclusion: The study adds to the understanding of the roles of 12R-LOX and TG activity in CE maturation and gives further insight into the effect of glycerol on the SC.
Contexte: Les kératinocytes à différenciation terminale acquièrent les enveloppes protéiniques des cornéocytes (ECP) complexées aux enveloppes lipidiques des cornéocytes (ELC). Ces deux composants structurels des enveloppes cornéocytaires (EC) subissent un processus de maturation en gagnant en hydrophobicité, en rigidité et en surface. Les linoléoyl-acyle-céramides sont traités par 12R-lipoxygénase (12R-LOX) et d’autres enzymes avant que la transglutaminase (TG) ne fixe les x-hydroxy-céramides à l’involucrine dans les ECP. Les protéines structurelles sont simultanément réticulées par la TG qui a été activée par la cathepsine D (CathD).
Objectifs: L’objectif principal de ces travaux visait à démontrer l’impact de l’humidité relative (HR) pendant la maturation de l’EC ex vivo. Des humidités relatives faible, optimale et élevée ont été retenues pour étudier l’effet des inhibiteurs de la protéase (IP) sur la maturation de l’EC et l’activité de la TG ; l’activité de CathD et 12R-LOX a également été mesurée à une HR optimale. Finalement, l’effet du glycérol sur la maturation de l’EC ex vivo a été testé à des humidités relatives faible, optimale et élevée. MÉTHODES: La première et neuvième bandes adhésives sur un site à l’arrière de l’oreille protégé de la lumière (photo-protégé, PP) et sur une joue exposée à la lumière (photo-exposée, PE) de volontaires sains ont été sélectionnées. La maturation de l’EC ex vivo a été évaluée par l’approche de la maturité relative d’EC (RCEM) reposant sur l’hydrophobicité et la rigidité de l’EC. Les deuxième et huitième bandes ont été exposées à l’humidité relative en présence d’inhibiteurs. RÉSULTATS: Indépendamment de la profondeur de bande adhésive, les EC des échantillons EP ont atteint la rigidité d’EC de la même manière que les EC matures du site PP, mais ces améliorations faisaient défaut en ce qui concerne l’hydrophobicité des EC. Une HR à 70 % était optimale pour la maturation de l’EC ex vivo. L’inhibition de l’activité du 12R-LOX a entraîné une rigidité accrue de l’EC, laquelle était réduite par l’inhibiteur de la TG. L’hydrophobicité des EC est restée inchangée pendant la maturation ex vivo en présence de l’inhibition de la TG ou du 12R-LOX. L’hydrophobicité des EC a été améliorée en présence de glycérol à une HR de 44 % et à une HR de 100 %, mais non à une HR de 70 %. L’activité de la TG a par ailleurs significativement diminué à une HR de 100 % par rapport à l’inhibiteur commercial LDN-27219. Cependant, un mélange inhibiteur de la protéase a inversé l’effet négatif de la surhydratation.
Conclusion: L’étude renforce la compréhension des rôles de l’activité de la TG et du 12R-LOX dans la maturation de l’EC et donne de plus amples détails sur l’effet du glycérol sur la couche cornée (stratum corneum, SC).
Keywords: enzymolgy; protease inhibition; rather; stratum corneum.
© 2019 The Authors. International Journal of Cosmetic Science published by John Wiley & Sons Ltd on behalf of Society of Cosmetic Scientists and Société Française de Cosmétologie.
Conflict of interest statement
RV is an employee of DSM Nutritional Products Ltd. AVR is a consultant to DSM Nutritional Products Ltd.
Figures






References
-
- Candi, E. , Schmidt, R. and Melino, G. The cornified envelope: a model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 6, 328–340 (2005). - PubMed
-
- Fluhr, J.W. , Kao, J. , Ahn, S.K. , Feingold, K.R. , Elias, P.M. and Jain, M. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J. Invest. Dermatol. 117, 44–51 (2001). - PubMed
-
- Wertz, P.W. and van den Bergh, B. The physical, chemical and functional properties of lipids in the skin and other biological barriers. Chem. Phys. Lipids 91, 85–96 (1998). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

