PD-L1 expression, CD8+ and CD4+ lymphocyte rate are predictive of pathological complete response after neoadjuvant chemoradiotherapy for squamous cell cancer of the thoracic esophagus
- PMID: 31429521
- PMCID: PMC6792480
- DOI: 10.1002/cam4.2359
PD-L1 expression, CD8+ and CD4+ lymphocyte rate are predictive of pathological complete response after neoadjuvant chemoradiotherapy for squamous cell cancer of the thoracic esophagus
Abstract
Background: Neoadjuvant chemoradiotherapy (CTRT) can effectively downstage esophageal squamous cell carcinoma (SCC) in patients with locally advanced disease and prolonged survival have been observed in patients with a pathological complete response (ypCR).
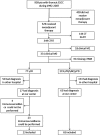
Aims and methods: This exploratory study aimed to identify immunological predictors of pCR after neoadjuvant CTRT within SCC microenvironment. The tumor regression after neoadjuvant therapy was measured according to the Mandard score system. Eighty-eight consecutive patients with SCC of the thoracic esophagus who received neoadjuvant CTRT were included in this retrospective study. Inclusion criteria were neoadjuvant CTRT and the availability of representative histological samples taken at diagnosis. We investigated immunohistochemical expression of CD4, Tbet, FoxP3, CD8, CD80, PD-L1, and PD-1, in the pretreatment biopsies and correlated the immunohistochemical profiles to patients' outcomes.
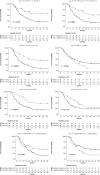
Results: After neoadjuvant CTRT, 23 patients had pCR, while 65 ones had partial response, stable disease or progression. PD-L1 expression and CD8+ and CD4+ lymphocyte rate were significantly higher in patients who had ypCR compared to those who had not (10 (0-55) vs 0 (0-0), P = 0.004, 73 (36-147) vs 21 (7-47), P = 0.0006 and 39 (23-74) vs 5 (0-13), P < 0.0001 respectively). The accuracy of expression of PD-L1+, CD8+, and CD4+ lymphocyte rate in identifying responders was AUC = 0.76 (P = 0.001), AUC = 0.81 (P = 0.0001) and AUC = 0.75 (P = 0.0001), respectively. Within the ypCR group, all patients with high infiltration of CD4+ T cell recurred/relapsed while only the 38.9% of those with low CD4+ T cell infiltration did the same (P = 0.058).
Conclusions: PD-L1 expression and CD8+ and CD4+ lymphocyte rate were predictive of ypCR after neoadjuvant CTRT for SCC of the thoracic esophagus with adequate accuracy. Furthermore, recurrence/relapse was associated with high level of CD4+ T cell infiltration. However, the small sample size prevented to draw definitive conclusions; further studies are necessary to evaluate the prognostic role of these markers.
Keywords: esophageal cancer; induction chemoradiotherapy; neoadjuvant therapy; squamous cell carcinoma; survival analysis.
© 2019 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
None to declare.
Figures


References
-
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. - PubMed
-
- Refaely Y, Krasna MJ. Multimodality therapy for esophageal cancer. Surg Clin North Am. 2002;82:729–746. - PubMed
-
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349(23):2241–2252. - PubMed
-
- Wu PC, Posner MC. The role of surgery in the management of oesophageal cancer. Lancet Oncol. 2003;4:481–488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

