Retinal ischemia induces α-SMA-mediated capillary pericyte contraction coincident with perivascular glycogen depletion
- PMID: 31429795
- PMCID: PMC6701129
- DOI: 10.1186/s40478-019-0761-z
Retinal ischemia induces α-SMA-mediated capillary pericyte contraction coincident with perivascular glycogen depletion
Abstract
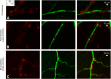
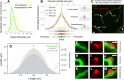
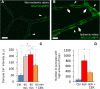
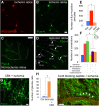
Increasing evidence indicates that pericytes are vulnerable cells, playing pathophysiological roles in various neurodegenerative processes. Microvascular pericytes contract during cerebral and coronary ischemia and do not relax after re-opening of the occluded artery, causing incomplete reperfusion. However, the cellular mechanisms underlying ischemia-induced pericyte contraction, its delayed emergence, and whether it is pharmacologically reversible are unclear. Here, we investigate i) whether ischemia-induced pericyte contractions are mediated by alpha-smooth muscle actin (α-SMA), ii) the sources of calcium rise in ischemic pericytes, and iii) if peri-microvascular glycogen can support pericyte metabolism during ischemia. Thus, we examined pericyte contractility in response to retinal ischemia both in vivo, using adaptive optics scanning light ophthalmoscopy and, ex vivo, using an unbiased stereological approach. We found that microvascular constrictions were associated with increased calcium in pericytes as detected by a genetically encoded calcium indicator (NG2-GCaMP6) or a fluoroprobe (Fluo-4). Knocking down α-SMA expression with RNA interference or fixing F-actin with phalloidin or calcium antagonist amlodipine prevented constrictions, suggesting that constrictions resulted from calcium- and α-SMA-mediated pericyte contractions. Carbenoxolone or a Cx43-selective peptide blocker also reduced calcium rise, consistent with involvement of gap junction-mediated mechanisms in addition to voltage-gated calcium channels. Pericyte calcium increase and capillary constrictions became significant after 1 h of ischemia and were coincident with depletion of peri-microvascular glycogen, suggesting that glucose derived from glycogen granules could support pericyte metabolism and delay ischemia-induced microvascular dysfunction. Indeed, capillary constrictions emerged earlier when glycogen breakdown was pharmacologically inhibited. Constrictions persisted despite recanalization but were reversible with pericyte-relaxant adenosine administered during recanalization. Our study demonstrates that retinal ischemia, a common cause of blindness, induces α-SMA- and calcium-mediated persistent pericyte contraction, which can be delayed by glucose driven from peri-microvascular glycogen. These findings clarify the contractile nature of capillary pericytes and identify a novel metabolic collaboration between peri-microvascular end-feet and pericytes.
Keywords: Alpha-smooth muscle actin; Capillary constriction; Retinal ischemia/reperfusion; Retinal pericytes; Retinal vasculature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Rouget C. Memoire sur le developpement, la structure et les proprietes physiologiques des capillaries sanguins et lymphatiques. Arch Physiol Norm Path. 1873;5:603–663.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

