Effect of Continuing Olanzapine vs Placebo on Relapse Among Patients With Psychotic Depression in Remission: The STOP-PD II Randomized Clinical Trial
- PMID: 31429896
- PMCID: PMC6704758
- DOI: 10.1001/jama.2019.10517
Effect of Continuing Olanzapine vs Placebo on Relapse Among Patients With Psychotic Depression in Remission: The STOP-PD II Randomized Clinical Trial
Abstract
Importance: Psychotic depression is a severely disabling and potentially lethal disorder. Little is known about the efficacy and tolerability of continuing antipsychotic medication for patients with psychotic depression in remission.
Objective: To determine the clinical effects of continuing antipsychotic medication once an episode of psychotic depression has responded to combination treatment with an antidepressant and antipsychotic agent.
Design, setting, and participants: Thirty-six week randomized clinical trial conducted at 4 academic medical centers. Patients aged 18 years or older had an episode of psychotic depression acutely treated with sertraline plus olanzapine for up to 12 weeks and met criteria for remission of psychosis and remission or near-remission of depressive symptoms for 8 weeks before entering the clinical trial. The study was conducted from November 2011 to June 2017, and the final date of follow-up was June 13, 2017.
Interventions: Participants were randomized either to continue olanzapine (n = 64) or switch from olanzapine to placebo (n = 62). All participants continued sertraline.
Main outcomes and measures: The primary outcome was risk of relapse. Main secondary outcomes were change in weight, waist circumference, lipids, serum glucose, and hemoglobin A1c (HbA1c).
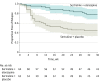
Results: Among 126 participants who were randomized (mean [SD] age, 55.3 years [14.9 years]; 78 women [61.9%]), 114 (90.5%) completed the trial. At the time of randomization, the median dosage of sertraline was 150 mg/d (interquartile range [IQR], 150-200 mg/d) and the median dosage of olanzapine was 15 mg/d (IQR, 10-20 mg/d). Thirteen participants (20.3%) randomized to olanzapine and 34 (54.8%) to placebo experienced a relapse (hazard ratio, 0.25; 95% CI, 0.13 to 0.48; P < .001). The effect of olanzapine on the daily rate of anthropometric and metabolic measures significantly differed from placebo for weight (0.13 lb; 95% CI, 0.11 to 0.15), waist circumference (0.009 inches; 95% CI, 0.004 to 0.014), and total cholesterol (0.29 mg/dL; 95% CI, 0.13 to 0.45) but was not significantly different for low-density lipoprotein cholesterol (0.04 mg/dL; 95% CI, -0.01 to 0.10), high-density lipoprotein cholesterol (-0.01 mg/dL; 95% CI, -0.03 to 0.01), triglyceride (-0.153 mg/dL; 95% CI, -0.306 to 0.004), glucose (-0.02 mg/dL; 95% CI, -0.12 to 0.08), or HbA1c levels (-0.0002 mg/dL; 95% CI, -0.0021 to 0.0016).
Conclusions and relevance: Among patients with psychotic depression in remission, continuing sertraline plus olanzapine compared with sertraline plus placebo reduced the risk of relapse over 36 weeks. This benefit needs to be balanced against potential adverse effects of olanzapine, including weight gain.
Trial registration: ClinicalTrials.gov Identifier: NCT01427608.
Conflict of interest statement
Figures
Comment in
-
Maintenance Treatment for Psychotic Depressive Disorders: Progress and Remaining Challenges.JAMA. 2019 Aug 20;322(7):615-617. doi: 10.1001/jama.2019.9682. JAMA. 2019. PMID: 31429877 No abstract available.
-
Continuing Antipsychotic Medication for Patients With Psychotic Depression in Remission.JAMA. 2019 Dec 24;322(24):2443. doi: 10.1001/jama.2019.17691. JAMA. 2019. PMID: 31860037 No abstract available.
References
-
- Farahani A, Correll CU. Are antipsychotics or antidepressants needed for psychotic depression? a systematic review and meta-analysis of trials comparing antidepressant or antipsychotic monotherapy with combination treatment. J Clin Psychiatry. 2012;73(4):486-496. doi:10.4088/JCP.11r07324 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

