Exploring Structure-Activity Relationship in Tacrine-Squaramide Derivatives as Potent Cholinesterase Inhibitors
- PMID: 31430943
- PMCID: PMC6723352
- DOI: 10.3390/biom9080379
Exploring Structure-Activity Relationship in Tacrine-Squaramide Derivatives as Potent Cholinesterase Inhibitors
Abstract
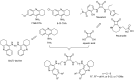
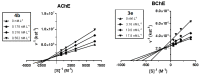
Tacrine was the first drug to be approved for Alzheimer's disease (AD) treatment, acting as a cholinesterase inhibitor. The neuropathological hallmarks of AD are amyloid-rich senile plaques, neurofibrillary tangles, and neuronal degeneration. The portfolio of currently approved drugs for AD includes acetylcholinesterase inhibitors (AChEIs) and N-methyl-d-aspartate (NMDA) receptor antagonist. Squaric acid is a versatile structural scaffold capable to be easily transformed into amide-bearing compounds that feature both hydrogen bond donor and acceptor groups with the possibility to create multiple interactions with complementary sites. Considering the relatively simple synthesis approach and other interesting properties (rigidity, aromatic character, H-bond formation) of squaramide motif, we combined this scaffold with different tacrine-based derivatives. In this study, we developed 21 novel dimers amalgamating squaric acid with either tacrine, 6-chlorotacrine or 7-methoxytacrine representing various AChEIs. All new derivatives were evaluated for their anti-cholinesterase activities, cytotoxicity using HepG2 cell line and screened to predict their ability to cross the blood-brain barrier. In this contribution, we also report in silico studies of the most potent AChE and BChE inhibitors in the active site of these enzymes.
Keywords: 6-chlorotacrine; 7-methoxytacrine; Alzheimer’s disease; bis(7)-tacrine; cholinesterases; in silico; in vitro; squaramides; tacrine.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures


References
-
- Zemek F., Drtinova L., Nepovimova E., Sepsova V., Korabecny J., Klimes J., Kuca K. Outcomes of Alzheimer’s disease therapy with acetylcholinesterase inhibitors and memantine. Expert Opin. Drug Saf. 2014;13:759–774. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

