The plasma membrane as a mechanochemical transducer
- PMID: 31431176
- PMCID: PMC6627014
- DOI: 10.1098/rstb.2018.0221
The plasma membrane as a mechanochemical transducer
Abstract
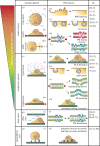
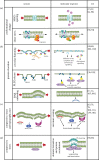
Cells are constantly submitted to external mechanical stresses, which they must withstand and respond to. By forming a physical boundary between cells and their environment that is also a biochemical platform, the plasma membrane (PM) is a key interface mediating both cellular response to mechanical stimuli, and subsequent biochemical responses. Here, we review the role of the PM as a mechanosensing structure. We first analyse how the PM responds to mechanical stresses, and then discuss how this mechanical response triggers downstream biochemical responses. The molecular players involved in PM mechanochemical transduction include sensors of membrane unfolding, membrane tension, membrane curvature or membrane domain rearrangement. These sensors trigger signalling cascades fundamental both in healthy scenarios and in diseases such as cancer, which cells harness to maintain integrity, keep or restore homeostasis and adapt to their external environment. This article is part of a discussion meeting issue 'Forces in cancer: interdisciplinary approaches in tumour mechanobiology'.
Keywords: mechanosensor; mechanotransduction; membrane tension; plasma membrane.
Conflict of interest statement
We declare we have no competing interests.
Figures
References
-
- Northey JJ, Przybyla L, Weaver VM. 2017. Tissue force programs cell fate and tumor aggression. Cancer Discovery 7, 1224–1237. (10.1158/2159-8290.CD-16-0733) - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
