Mitofusins modulate the increase in mitochondrial length, bioenergetics and secretory phenotype in therapy-induced senescent melanoma cells
- PMID: 31431479
- PMCID: PMC6735661
- DOI: 10.1042/BCJ20190405
Mitofusins modulate the increase in mitochondrial length, bioenergetics and secretory phenotype in therapy-induced senescent melanoma cells
Abstract
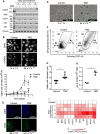
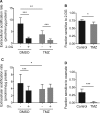
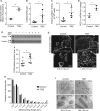
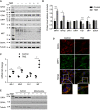
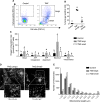
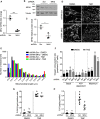
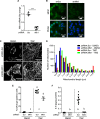
Cellular senescence is an endpoint of chemotherapy, and targeted therapies in melanoma and the senescence-associated secretory phenotype (SASP) can affect tumor growth and microenvironment, influencing treatment outcomes. Metabolic interventions can modulate the SASP, and an enhanced mitochondrial energy metabolism supports resistance to therapy in melanoma cells. Herein, we assessed the mitochondrial function of therapy-induced senescent melanoma cells obtained after exposing the cells to temozolomide (TMZ), a methylating chemotherapeutic agent. Senescence induction in melanoma was accompanied by a substantial increase in mitochondrial basal, ATP-linked, and maximum respiration rates and in coupling efficiency, spare respiratory capacity, and respiratory control ratio. Further examinations revealed an increase in mitochondrial mass and length. Alterations in mitochondrial function and morphology were confirmed in isolated senescent cells, obtained by cell-size sorting. An increase in mitofusin 1 and 2 (MFN1 and 2) expression and levels was observed in senescent cells, pointing to alterations in mitochondrial fusion. Silencing mitofusin expression with short hairpin RNA (shRNA) prevented the increase in mitochondrial length, oxygen consumption rate and secretion of interleukin 6 (IL-6), a component of the SASP, in melanoma senescent cells. Our results represent the first in-depth study of mitochondrial function in therapy-induced senescence in melanoma. They indicate that senescence increases mitochondrial mass, length and energy metabolism; and highlight mitochondria as potential pharmacological targets to modulate senescence and the SASP.
Keywords: bioenergetics; cell senescence; hemotherapy; melanoma; mitochondria; mitofusin.
© 2019 The Author(s).
Conflict of interest statement
The Authors declare that there are no competing interests associated with the manuscript.
Figures

Similar articles
-
Mitofusin 1 silencing decreases the senescent associated secretory phenotype, promotes immune cell recruitment and delays melanoma tumor growth after chemotherapy.Sci Rep. 2024 Jan 9;14(1):909. doi: 10.1038/s41598-024-51427-7. Sci Rep. 2024. PMID: 38195762 Free PMC article.
-
Oncogene-induced senescence mitochondrial metabolism and bioenergetics drive the secretory phenotype: further characterization and comparison with other senescence-inducing stimuli.Redox Biol. 2025 May;82:103606. doi: 10.1016/j.redox.2025.103606. Epub 2025 Mar 22. Redox Biol. 2025. PMID: 40158257 Free PMC article.
-
Senescence-associated secretory factors induced by cisplatin in melanoma cells promote non-senescent melanoma cell growth through activation of the ERK1/2-RSK1 pathway.Cell Death Dis. 2018 Feb 15;9(3):260. doi: 10.1038/s41419-018-0303-9. Cell Death Dis. 2018. PMID: 29449532 Free PMC article.
-
Mitofusins, from Mitochondria to Metabolism.Mol Cell. 2016 Mar 3;61(5):683-694. doi: 10.1016/j.molcel.2016.02.022. Mol Cell. 2016. PMID: 26942673 Review.
-
Bioenergetic roles of mitochondrial fusion.Biochim Biophys Acta. 2016 Aug;1857(8):1277-1283. doi: 10.1016/j.bbabio.2016.04.002. Epub 2016 Apr 7. Biochim Biophys Acta. 2016. PMID: 27060252 Review.
Cited by
-
Mesenchymal Stromal Cells From Emphysematous Donors and Their Extracellular Vesicles Are Unable to Reverse Cardiorespiratory Dysfunction in Experimental Severe Emphysema.Front Cell Dev Biol. 2021 May 31;9:661385. doi: 10.3389/fcell.2021.661385. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34136481 Free PMC article.
-
Mechanisms of RPE senescence and potential role of αB crystallin peptide as a senolytic agent in experimental AMD.Exp Eye Res. 2022 Feb;215:108918. doi: 10.1016/j.exer.2021.108918. Epub 2022 Jan 2. Exp Eye Res. 2022. PMID: 34986369 Free PMC article.
-
Mitochondrial metabolism determines the functional status of human sperm and correlates with semen parameters.Front Cell Dev Biol. 2022 Aug 30;10:926684. doi: 10.3389/fcell.2022.926684. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36111336 Free PMC article.
-
Subcellular structure, heterogeneity, and plasticity of senescent cells.Aging Cell. 2024 Apr;23(4):e14154. doi: 10.1111/acel.14154. Epub 2024 Mar 30. Aging Cell. 2024. PMID: 38553952 Free PMC article. Review.
-
Persistent accumulation of therapy-induced senescent cells: an obstacle to long-term cancer treatment efficacy.Int J Oral Sci. 2025 Aug 1;17(1):59. doi: 10.1038/s41368-025-00380-w. Int J Oral Sci. 2025. PMID: 40750580 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

