Artesunate attenuates LPS-induced osteoclastogenesis by suppressing TLR4/TRAF6 and PLCγ1-Ca2+-NFATc1 signaling pathway
- PMID: 31431733
- PMCID: PMC7468527
- DOI: 10.1038/s41401-019-0289-6
Artesunate attenuates LPS-induced osteoclastogenesis by suppressing TLR4/TRAF6 and PLCγ1-Ca2+-NFATc1 signaling pathway
Abstract
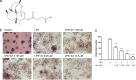
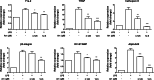
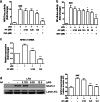
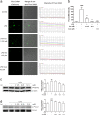
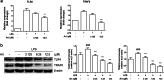
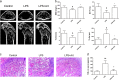
In chronic infectious diseases caused by gram-negative bacteria, such as osteomyelitis, septic arthritis, and periodontitis, osteoclastic activity is enhanced with elevated inflammation, which disturbs the bone homeostasis and results in osteolysis. Lipopolysaccharide (LPS), as a bacteria product, plays an important role in this process. Recent evidence shows that an antimalarial drug artesunate attenuates LPS-induced osteolysis independent of RANKL. In this study we evaluated the effects of artesunate on LPS-induced osteoclastogenesis in vitro and femur osteolysis in vivo, and explored the mechanisms underlying the effects of artesunate on LPS-induced osteoclast differentiation independent of RANKL. In preosteoclastic RAW264.7 cells, we found that artesunate (1.56-12.5 μM) dose dependently inhibited LPS-induced osteoclast formation accompanied by suppressing LPS-stimulated osteoclast-related gene expression (Fra-2, TRAP, Cathepsin K, β3-integrin, DC-STAMP, and Atp6v0d2). We showed that artesunate (3.125-12.5 µM) inhibited LPS-stimulated nuclear factor of activated T cells c1 (NFATc1) but not NF-κB transcriptional activity; artesunate (6.25, 12.5 μM) significantly inhibited LPS-stimulated NFATc1 protein expression. Furthermore, artesunate treatment markedly suppressed LPS-induced Ca2+ influx, and decreased the expression of PP2B-Aα (calcineurin) and pPLCγ1 in the cells. In addition, artesunate treatment significantly decreased the expression of upstream signals TLR4 and TRAF6 during LPS-induced osteoclastogenesis. Administration of artesunate (10 mg/kg, ip) for 8 days effectively inhibited serum TNF-α levels and ameliorated LPS (5 mg/kg, ip)-induced inflammatory bone loss in vivo. Taken together, artesunate attenuates LPS-induced inflammatory osteoclastogenesis by inhibiting the expression of TLR4/TRAF6 and the downstream PLCγ1-Ca2+-NFATc1 signaling pathway. Artesunate is a valuable choice to treat bone loss induced by gram-negative bacteria infection or inflammation in RANKL-independent pathway.
Keywords: Ca2+; LPS; NFATc1; PP2B-Aα; RAW264.7 cells; TLR4; artesunate; chronic infectious diseases; osteoclast.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

