Long-term effectiveness of tumour necrosis factor-α inhibitor treatment for psoriatic arthritis in the UK: a multicentre retrospective study
- PMID: 31431979
- PMCID: PMC6649900
- DOI: 10.1093/rap/rky042
Long-term effectiveness of tumour necrosis factor-α inhibitor treatment for psoriatic arthritis in the UK: a multicentre retrospective study
Abstract
Objective: Real-world evidence of the long-term effectiveness of TNF-α inhibitor (TNFi) therapy in patients with PsA is limited. This study was conducted to describe patterns of TNFi therapy and treatment responses in patients with PsA treated in UK clinical practice.
Methods: A multicentre, retrospective, observational cohort study of consenting patients treated with TNFi for PsA with ≥3 years follow-up from first TNFi initiation (observation period) was carried out in 11 UK National Health Service hospitals. Data were collected concerning baseline patient characteristics, PsA-related treatment pathways and TNFi treatment responses (PsA response criteria components: swollen/tender joint counts, physician and patient global assessments).
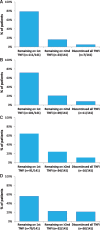
Results: The mean age of patients (n = 141) was 50.3 (s.d.: 12.1) years (50% male). During a median observation period of 4.5 (range: 3.4-5.5) years, patients received a median of one (range: one to five) TNFi. Twelve-week response rates for first TNFi (where available) were as follows: 80% (n = 64/80) for swollen joint counts, 79% (n = 63/79) for tender joint counts, 79% (n = 37/47) for physician global assessments, 69% (n = 41/59) for patient global assessments and 79% (n = 37/47) for PsA response criteria. At the end of the observation period, the proportions of patients remaining on first, second, third and fourth/fifth TNFi were 56, 15, 5 and 3%, respectively; 21% of patients permanently discontinued TNFi therapy.
Conclusion: Long-term TNFi therapy is generally well tolerated and may be effective; however, after initial TNFi failure, there appears to be progressively less benefit and more adverse effects with successive TNFi switches. Strategies are needed for effective therapy for PsA beyond the first TNFi failure.
Keywords: observational study; patient global assessment; physician global assessment; psoriatic arthritis; swollen joints; tender joints; treatment persistence; tumour necrosis factor inhibitors.
Figures



References
-
- Huynh D, Kavanaugh A.. Psoriatic arthritis: current therapy and future approaches. Rheumatology 2015;54:20–8. - PubMed
-
- NICE. Etanercept, Infliximab and Adalimumab for the Treatment of Psoriatic Arthritis: Guidance and Guidelines (TA199). 2010. http://www.nice.org.uk/guidance/ta199 (1 February 2016, date last accessed).
-
- Olivieri I, D'Angelo S, Palazzi C, Padula A.. Advances in the management of psoriatic arthritis. Nat Rev Rheumatol 2014;10:531–42. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
