Quantitative and kinetic analyses of peanut allergens as affected by food processing
- PMID: 31432004
- PMCID: PMC6694862
- DOI: 10.1016/j.fochx.2019.100004
Quantitative and kinetic analyses of peanut allergens as affected by food processing
Abstract
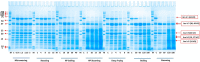
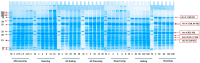
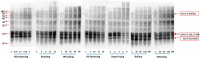
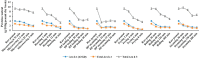
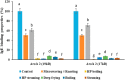
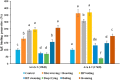
Peanuts contain four major allergens with differences in allergenic potency. Thermal processing can influence the allergenic properties of peanuts. Until now, a kinetic model has not been reported to assess the changes of soluble allergen (extracted from processed peanuts) content as affected by various thermal processing methods. Our objective is to characterize the reaction kinetics of the thermal processing methods, including wet processing (boiling with/without high-pressure, steaming with/without high-pressure), deep-frying and dry processing (microwaving and roasting) using five time intervals. The relationships between processing time and extractable major allergen content could be explained by a simple linear regression kinetic model (except high-pressure steaming). Among all the methods with optimal processing point, frying for 6 min had a relatively lower IgE binding (linear epitopes) ratio, possibly due to the processing conditions, which caused break down, cross-linking and aggregation of Ara h 2, and a relatively lower solubility.
Keywords: IgE-binding properties; Kinetic analysis; Peanut allergen; Protein denaturation; Proximate analysis; SDS-sample-buffer-soluble protein; Thermal processing; Water-soluble protein.
Figures




References
-
- AOAC International . 19th ed. Association of Official Analytical Chemists; Gaitherburg, MD: 2012. Official methods of analysis of AOAC international.
-
- Beyer K., Morrowa E., Li X.M., Bardina L., Bannon G.A., Burks A.W., Sampson H.A. Effects of cooking methods on peanut allergenicity. Journal of Allergy and Clinical Immunology. 2001;107:1077–1081. - PubMed
-
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. - PubMed
-
- Bu G., Luo Y., Zheng Z., Zheng H. Effect of heat treatment on the antigenicity of bovine α-lactalbumin and β-lactoglobulin in whey protein isolate. Food and Agricultural Immunology. 2009;20:195–206.
LinkOut - more resources
Full Text Sources

