Pro-oxidant and lifespan extension effects of caffeine and related methylxanthines in Caenorhabditis elegans
- PMID: 31432005
- PMCID: PMC6694850
- DOI: 10.1016/j.fochx.2019.100005
Pro-oxidant and lifespan extension effects of caffeine and related methylxanthines in Caenorhabditis elegans
Erratum in
-
Erratum regarding missing Declaration of Competing Interest statements in previously published articles.Food Chem X. 2021 Jan 18;12:100111. doi: 10.1016/j.fochx.2020.100111. eCollection 2021 Dec 30. Food Chem X. 2021. PMID: 35024606 Free PMC article.
Abstract
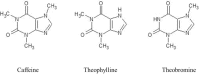
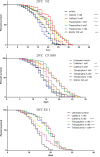
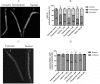
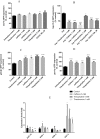
Caffeine and related purine alkaloids are common ingredients of many stimulating drinks. Studies have shown that lower concentrations of caffeine have a protective role in aging-related disorders. However, the associated mode of action of caffeine and its related methylxanthines is still not clear. In this study, we demonstrated that caffeine and theophylline promote longevity in Caenorhabditis elegans. Lifespan studies with the wild type, DAF-16 and SKN-1 mutant strains indicated that the methylxanthines-mediated lifespan extension in C. elegans was independent of DAF-16/FOXO and SKN-1. All the tested methylxanthines could protect C. elegans against acute oxidative stress. At early stages of life, an increase of ROS (reactive oxygen species) induced the translocation of DAF-16 and SKN-1, resulting in upregulation of several antioxidant genes, for example, sod-3p::GFP, gst-4p::GFP, gcs-1p::GFP; and downregulation of hsp-16.2p::GFP. RT-PCR corroborates the upregulation of gst-4 and skn-1 genes. The expression of DAF-16 decreased although its nuclear translocation was induced.
Keywords: Caenorhabditis elegans; Caffeine; DAF-16/FOXO; Pro-oxidant effect; SKN-1; Theobromine; Theophylline.
Figures

Similar articles
-
Elucidation of the nematicidal mode of action of grammicin on Caenorhabditis elegans.Pestic Biochem Physiol. 2022 Nov;188:105244. doi: 10.1016/j.pestbp.2022.105244. Epub 2022 Sep 19. Pestic Biochem Physiol. 2022. PMID: 36464355
-
Hibiscus sabdariffa L. extract prolongs lifespan and protects against amyloid-β toxicity in Caenorhabditis elegans: involvement of the FoxO and Nrf2 orthologues DAF-16 and SKN-1.Eur J Nutr. 2020 Feb;59(1):137-150. doi: 10.1007/s00394-019-01894-w. Epub 2019 Feb 1. Eur J Nutr. 2020. PMID: 30710163
-
2-Butoxytetrahydrofuran and Palmitic Acid from Holothuria scabra Enhance C. elegans Lifespan and Healthspan via DAF-16/FOXO and SKN-1/NRF2 Signaling Pathways.Pharmaceuticals (Basel). 2022 Nov 9;15(11):1374. doi: 10.3390/ph15111374. Pharmaceuticals (Basel). 2022. PMID: 36355546 Free PMC article.
-
Capsaicinoid-Glucosides of Fresh Hot Pepper Promotes Stress Resistance and Longevity in Caenorhabditis elegans.Plant Foods Hum Nutr. 2022 Mar;77(1):30-36. doi: 10.1007/s11130-021-00939-y. Epub 2022 Feb 4. Plant Foods Hum Nutr. 2022. PMID: 35119578
-
Glochidion zeylanicum leaf extracts exhibit lifespan extending and oxidative stress resistance properties in Caenorhabditis elegans via DAF-16/FoxO and SKN-1/Nrf-2 signaling pathways.Phytomedicine. 2019 Nov;64:153061. doi: 10.1016/j.phymed.2019.153061. Epub 2019 Jul 31. Phytomedicine. 2019. PMID: 31401497
Cited by
-
Pediococcus acidilactici Promotes the Longevity of C. elegans by Regulating the Insulin/IGF-1 and JNK/MAPK Signaling, Fat Accumulation and Chloride Ion.Front Nutr. 2022 Apr 1;9:821685. doi: 10.3389/fnut.2022.821685. eCollection 2022. Front Nutr. 2022. PMID: 35433778 Free PMC article.
-
Maternal Caffeine Intake Disrupts Eggshell Integrity and Retards Larval Development by Reducing Yolk Production in a Caenorhabditis elegans Model.Nutrients. 2020 May 7;12(5):1334. doi: 10.3390/nu12051334. Nutrients. 2020. PMID: 32392893 Free PMC article.
-
Extension of chronological lifespan in Schizosaccharomyces pombe.Genes Cells. 2021 Jul;26(7):459-473. doi: 10.1111/gtc.12854. Epub 2021 May 12. Genes Cells. 2021. PMID: 33977597 Free PMC article. Review.
-
Simultaneous Quantification of Antioxidants Paraxanthine and Caffeine in Human Saliva by Electrochemical Sensing for CYP1A2 Phenotyping.Antioxidants (Basel). 2020 Dec 24;10(1):10. doi: 10.3390/antiox10010010. Antioxidants (Basel). 2020. PMID: 33374269 Free PMC article.
-
Alterations in Bacterial Metabolism Contribute to the Lifespan Extension Exerted by Guarana in Caenorhabditis elegans.Nutrients. 2022 May 9;14(9):1986. doi: 10.3390/nu14091986. Nutrients. 2022. PMID: 35565952 Free PMC article.
References
-
- Balaban R.S., Nemoto S., Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–495. - PubMed
-
- Barnes P.J. Theophylline. American Journal of Respiratory and Critical Care Medicine. 2013;188(8):901–906. - PubMed
-
- Bischof L.J., Huffman D.L., Aroian R.V. Assays for toxicity studies in C. elegans with Bt crystal proteins. Springer; 2006. pp. 139–154. - PubMed
-
- Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

