Bone sialoprotein-αvβ3 integrin axis promotes breast cancer metastasis to the bone
- PMID: 31432600
- PMCID: PMC6778634
- DOI: 10.1111/cas.14172
Bone sialoprotein-αvβ3 integrin axis promotes breast cancer metastasis to the bone
Abstract
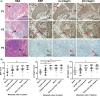
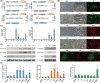
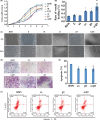
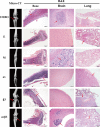
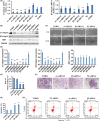
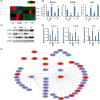
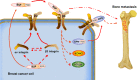
The underlying mechanisms of breast cancer cells metastasizing to distant sites are complex and multifactorial. Bone sialoprotein (BSP) and αvβ3 integrin were reported to promote the metastatic progress of breast cancer cells, particularly metastasis to bone. Most theories presume that BSP promotes breast cancer metastasis by binding to αvβ3 integrin. Interestingly, we found the αvβ3 integrin decreased in BSP silenced cells (BSPi), which have weak ability to form bone metastases. However, the relevance of their expression in primary tumor and the way they participate in metastasis are not clear. In this study, we evaluated the relationship between BSP, αvβ3 integrin levels, and the bone metastatic ability of breast cancer cells in patient tissues, and the data indicated that the αvβ3 integrin level is closely correlated to BSP level and metastatic potential. Overexpression of αvβ3 integrin in cancer cells could reverse the effect of BSPi in vitro and promote bone metastasis in a mouse model, whereas knockdown of αvβ3 integrin have effects just like BSPi. Moreover, The Cancer Genome Atlas data and RT-PCR analysis have also shown that SPP1, KCNK2, and PTK2B might be involved in this process. Thus, we propose that αvβ3 integrin is one of the downstream factors regulated by BSP in the breast cancer-bone metastatic cascade.
Keywords: bone sialoprotein; breast cancer; gene expression; metastasis; αvβ3 integrin.
© 2019 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures

Similar articles
-
BSP gene silencing inhibits migration, invasion, and bone metastasis of MDA-MB-231BO human breast cancer cells.PLoS One. 2013 May 7;8(5):e62936. doi: 10.1371/journal.pone.0062936. Print 2013. PLoS One. 2013. PMID: 23667544 Free PMC article.
-
Bone sialoprotein and osteopontin in bone metastasis of osteotropic cancers.Crit Rev Oncol Hematol. 2014 Feb;89(2):330-41. doi: 10.1016/j.critrevonc.2013.08.013. Epub 2013 Sep 7. Crit Rev Oncol Hematol. 2014. PMID: 24071501 Free PMC article. Review.
-
Bone sialoprotein, matrix metalloproteinase 2, and alpha(v)beta3 integrin in osteotropic cancer cell invasion.J Natl Cancer Inst. 2004 Jun 16;96(12):956-65. doi: 10.1093/jnci/djh169. J Natl Cancer Inst. 2004. PMID: 15199115
-
[Inhibitory effect of bone sialoprotein silencing on the adhesion ability of breast cancer cells to bone matrix].Sheng Wu Gong Cheng Xue Bao. 2011 Feb;27(2):233-9. Sheng Wu Gong Cheng Xue Bao. 2011. PMID: 21650048 Chinese.
-
Expression of bone matrix proteins in human breast cancer: potential roles in microcalcification formation and in the genesis of bone metastases.Bull Cancer. 1997 Jan;84(1):17-24. Bull Cancer. 1997. PMID: 9180854 Review.
Cited by
-
The research progress into cellular mechanosensitive ion channels mediating cancer pain.Channels (Austin). 2025 Dec;19(1):2517109. doi: 10.1080/19336950.2025.2517109. Epub 2025 Jun 14. Channels (Austin). 2025. PMID: 40515752 Free PMC article. Review.
-
Animal models of cancer metastasis to the bone.Front Oncol. 2023 Apr 5;13:1165380. doi: 10.3389/fonc.2023.1165380. eCollection 2023. Front Oncol. 2023. PMID: 37091152 Free PMC article. Review.
-
Comparison of Linear vs. Cyclic RGD Pentapeptide Interactions with Integrin αvβ3 by Molecular Dynamics Simulations.Biology (Basel). 2021 Jul 20;10(7):688. doi: 10.3390/biology10070688. Biology (Basel). 2021. PMID: 34356543 Free PMC article.
-
New players in the landscape of renal cell carcinoma bone metastasis and therapeutic opportunities.Int J Cancer. 2025 Feb 1;156(3):475-487. doi: 10.1002/ijc.35181. Epub 2024 Sep 22. Int J Cancer. 2025. PMID: 39306698 Free PMC article. Review.
-
Breast Cancer Bone Metastasis: A Narrative Review of Emerging Targeted Drug Delivery Systems.Cells. 2022 Jan 24;11(3):388. doi: 10.3390/cells11030388. Cells. 2022. PMID: 35159207 Free PMC article. Review.
References
-
- Waltregny D, Bellahcène A, Castronovo V, et al. Prognostic value of bone sialoprotein expression in clinically localized human prostate cancer. J Natl Cancer Inst. 1998;90:1000‐1008. - PubMed
-
- Bellahcène A, Kroll M, Liebens F, Castronovo V. Bone sialoprotein expression in primary human breast cancer is associated with bone metastases development. J Bone Miner Res. 1996;11:665‐670. - PubMed
-
- Zhu B, Guo Z, Lin L, Liu Q. Serum BSP, PSADT, and Spondin‐2 levels in prostate cancer and the diagnostic significance of their ROC curves in bone metastasis. Eur Rev Med Pharmacol Sci. 2017;21:61‐67. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

