Assessment of Prognostic Value of Left Ventricular Global Longitudinal Strain for Early Prediction of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis
- PMID: 31433450
- PMCID: PMC6705141
- DOI: 10.1001/jamacardio.2019.2952
Assessment of Prognostic Value of Left Ventricular Global Longitudinal Strain for Early Prediction of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis
Abstract
Importance: Echocardiographic left ventricular global longitudinal strain (GLS) detects early subclinical ventricular dysfunction and can be used in patients receiving potentially cardiotoxic chemotherapy. A meta-analysis of the prognostic value of GLS for cancer therapy-related cardiac dysfunction (CTRCD) has not been performed, to our knowledge.
Objective: To explore the prognostic value of GLS for the prediction of CTRCD.
Data sources: Systematic search of the MEDLINE, Embase, Scopus, and the Cochrane Library databases from database inception to June 1, 2018.
Study selection: Cohort studies assessing the prognostic or discriminatory performance of GLS before or during chemotherapy for subsequent CTRCD.
Data extraction and synthesis: Random-effects meta-analysis and hierarchical summary receiver operating characteristic curves (HSROCs) were used to summarize the prognostic and discriminatory performance of different GLS indices. Publication bias was assessed using the Egger test, and meta-regression was performed to assess sources of heterogeneity.
Main outcomes and measures: The primary outcome was CTRCD, defined as a clinically significant change in left ventricular ejection fraction with or without new-onset heart failure symptoms.
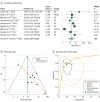
Results: Analysis included 21 studies comprising 1782 patients with cancer, including breast cancer, hematologic malignancies, or sarcomas, treated with anthracyclines with or without trastuzumab. The incidence of CTRCD ranged from 9.3% to 43.8% over a mean follow-up of 4.2 to 23.0 months (pooled incidence, 21.0%). For active treatment absolute GLS (9 studies), the high-risk cutoff values ranged from -21.0% to -13.8%, with worse GLS associated with a higher CTRCD risk (odds ratio, 12.27; 95% CI, 7.73-19.47; area under the HSROC, 0.86; 95% CI, 0.83-0.89). For relative changes vs a baseline value (9 studies), cutoff values ranged from 2.3% to 15.9%, with a greater decrease linked to a 16-fold higher risk of CTRCD (odds ratio, 15.82; 95% CI, 5.84-42.85; area under the HSROC, 0.86; 95% CI, 0.83-0.89). Both indices showed significant publication bias. Meta-regression identified differences in sample size and CTRCD definition but not GLS cutoff value as significant sources of interstudy heterogeneity.
Conclusions and relevance: In this meta-analysis, measurement of GLS after initiation of potentially cardiotoxic chemotherapy with anthracyclines with or without trastuzumab had good prognostic performance for subsequent CTRCD. However, risk of bias in the original studies, publication bias, and limited data on the incremental value of GLS and its optimal cutoff values highlight the need for larger prospective multicenter studies.
Conflict of interest statement
Figures



References
-
- Plana JC, Galderisi M, Barac A, et al. . Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911-939. doi:10.1016/j.echo.2014.07.012 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

