Therapeutic Effect of Targeting Branched-Chain Amino Acid Catabolic Flux in Pressure-Overload Induced Heart Failure
- PMID: 31433721
- PMCID: PMC6585363
- DOI: 10.1161/JAHA.118.011625
Therapeutic Effect of Targeting Branched-Chain Amino Acid Catabolic Flux in Pressure-Overload Induced Heart Failure
Abstract
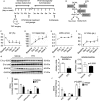
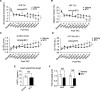
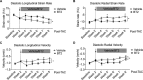
Background Branched-chain amino acid (BCAA) catabolic defect is an emerging metabolic hallmark in failing hearts in human and animal models. The therapeutic impact of targeting BCAA catabolic flux under pathological conditions remains understudied. Methods and Results BT2 (3,6-dichlorobenzo[b]thiophene-2-carboxylic acid), a small-molecule inhibitor of branched-chain ketoacid dehydrogenase kinase, was used to enhance BCAA catabolism. After 2 weeks of transaortic constriction, mice with significant cardiac dysfunctions were treated with vehicle or BT2. Serial echocardiograms showed continuing pathological deterioration in left ventricle of the vehicle-treated mice, whereas the BT2-treated mice showed significantly preserved cardiac function and structure. Moreover, BT2 treatment improved systolic contractility and diastolic mechanics. These therapeutic benefits appeared to be independent of impacts on left ventricle hypertrophy but associated with increased gene expression involved in fatty acid utilization. The BT2 administration showed no signs of apparent toxicity. Conclusions Our data provide the first proof-of-concept evidence for the therapeutic efficacy of restoring BCAA catabolic flux in hearts with preexisting dysfunctions. The BCAA catabolic pathway represents a novel and potentially efficacious target for treatment of heart failure.
Keywords: amino acids; heart failure; hypertrophy/remodeling; metabolism; therapy.
Figures

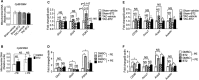
References
-
- Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. - PubMed
-
- Rajadurai J, Tse HF, Wang CH, Yang NI, Zhou J, Sim D. Understanding the epidemiology of heart failure to improve management practices: an Asia‐pacific perspective. J Card Fail. 2017;23:327–339. - PubMed
-
- Ezekowitz JA, Kaul P, Bakal JA, Quan H, McAlister FA. Trends in heart failure care: has the incident diagnosis of heart failure shifted from the hospital to the emergency department and outpatient clinics? Eur J Heart Fail. 2011;13:142–147. - PubMed
-
- Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation. 2007;116:434–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

