Innovative strategies for the elimination of viral hepatitis at a national level: A country case series
- PMID: 31433902
- PMCID: PMC6790606
- DOI: 10.1111/liv.14222
Innovative strategies for the elimination of viral hepatitis at a national level: A country case series
Abstract
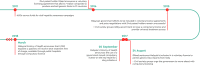
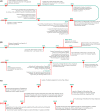
Viral hepatitis is a leading cause of morbidity and mortality worldwide, but has long been neglected by national and international policymakers. Recent modelling studies suggest that investing in the global elimination of viral hepatitis is feasible and cost-effective. In 2016, all 194 member states of the World Health Organization endorsed the goal to eliminate viral hepatitis as a public health threat by 2030, but complex systemic and social realities hamper implementation efforts. This paper presents eight case studies from a diverse range of countries that have invested in responses to viral hepatitis and adopted innovative approaches to tackle their respective epidemics. Based on an investment framework developed to build a global investment case for the elimination of viral hepatitis by 2030, national activities and key enablers are highlighted that showcase the feasibility and impact of concerted hepatitis responses across a range of settings, with different levels of available resources and infrastructural development. These case studies demonstrate the utility of taking a multipronged, public health approach to: (a) evidence-gathering and planning; (b) implementation; and (c) integration of viral hepatitis services into the Agenda for Sustainable Development. They provide models for planning, investment and implementation strategies for other countries facing similar challenges and resource constraints.
Keywords: developing countries; disease elimination; hepatitis B; hepatitis C; investment case; organizational case studies.
© 2019 The Authors. Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
AHS reports grants and travel funding to her institution from ViiV Healthcare. ETH is the former director of the Medicines Patent Pool. NS has received investigator‐initiated research funding from Gilead Sciences. JVL reports grants and personal fees from AbbVie, Gilead Sciences and MSD, personal fees from CEPHEID and Janssen outside the submitted work. MES is principal investigator in an investigator‐initiated trial sponsored by Gilead Sciences (received no PI fees, trial closed April 17th 2019) and reports an educational grant to travel to EASL 2019 (Gilead Sciences). SJH received honoraria from Gilead, unrelated to submitted work. MH's institute receives investigator‐initiated research funding from Gilead Sciences, Abbvie and BMS. JH received the Gilead Sciences Australia fellowship (2017). DW, CK, RA, RBL, MB, LA, AG, SH, RH, WL, RBM, SO, RP, MS, CWS, TS, MT, TW and ESS have nothing to declare.
Figures





Comment in
-
Colombian experience in the management of hepatitis C.Liver Int. 2020 Dec;40(12):3143. doi: 10.1111/liv.14582. Epub 2020 Jul 12. Liver Int. 2020. PMID: 32602224 No abstract available.
-
Authors' response to Letter to the Editor: 'Colombian experience in the management of hepatitis C'.Liver Int. 2020 Dec;40(12):3142-3143. doi: 10.1111/liv.14653. Liver Int. 2020. PMID: 32875663 No abstract available.
References
-
- World Health Organization . Global Hepatitis Report 2017. Geneva, Switzerland: World Health Organization; 2017.
-
- Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546‐1555. - PubMed
-
- World Health Organization . Global Hepatitis Report 2017. Geneva: WHO; 2017. [updated 2017; cited 2018 24 March]; Available from: http://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng....
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

