A Mass Spectrometry-Based Study Shows that Volatiles Emitted by Arthrobacter agilis UMCV2 Increase the Content of Brassinosteroids in Medicago truncatula in Response to Iron Deficiency Stress
- PMID: 31434211
- PMCID: PMC6719008
- DOI: 10.3390/molecules24163011
A Mass Spectrometry-Based Study Shows that Volatiles Emitted by Arthrobacter agilis UMCV2 Increase the Content of Brassinosteroids in Medicago truncatula in Response to Iron Deficiency Stress
Abstract
Iron is an essential plant micronutrient. It is a component of numerous proteins and participates in cell redox reactions; iron deficiency results in a reduction in nutritional quality and crop yields. Volatiles from the rhizobacterium Arthrobacter agilis UMCV2 induce iron acquisition mechanisms in plants. However, it is not known whether microbial volatiles modulate other metabolic plant stress responses to reduce the negative effect of iron deficiency. Mass spectrometry has great potential to analyze metabolite alterations in plants exposed to biotic and abiotic factors. Direct liquid introduction-electrospray-mass spectrometry was used to study the metabolite profile in Medicago truncatula due to iron deficiency, and in response to microbial volatiles. The putatively identified compounds belonged to different classes, including pigments, terpenes, flavonoids, and brassinosteroids, which have been associated with defense responses against abiotic stress. Notably, the levels of these compounds increased in the presence of the rhizobacterium. In particular, the analysis of brassinolide by gas chromatography in tandem with mass spectrometry showed that the phytohormone increased ten times in plants grown under iron-deficient growth conditions and exposed to microbial volatiles. In this mass spectrometry-based study, we provide new evidence on the role of A. agilis UMCV2 in the modulation of certain compounds involved in stress tolerance in M. truncatula.
Keywords: DLI-ESI-MS; Fe deficiency; legumes; microbial volatiles.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

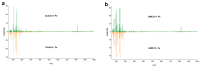
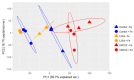

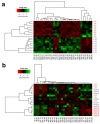

References
-
- Analytical Methods Committee AMCTB No. 81. A “periodic table” of mass spectrometry instrumentation and acronyms. Anal. Methods. 2017;9:5086–5090. - PubMed
-
- García-Flores M., Juárez-Colunga S., García-Casarrubias A., Trachsel S., Winkler R., Tiessen A. Metabolic profiling of plant extracts using direct-injection electrospray ionization mass spectrometry allows for high-throughput phenotypic characterization according to genetic and environmental effects. J. Agric. Food Chem. 2015;63:1042–1052. doi: 10.1021/jf504853w. - DOI - PubMed
-
- Gamboa-Becerra R., Montero-Vargas J., Martínez-Jarquín S., Gálvez-Ponce E., Moreno-Pedraza A., Winkler R. Rapid classification of coffee products by data mining models from direct electrospray and plasma-based mass spectrometry analyses. Food Anal. Methods. 2016;10:1359–1368. doi: 10.1007/s12161-016-0696-y. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

