Lipolytic Effects of 3-Iodothyronamine (T1AM) and a Novel Thyronamine-Like Analog SG-2 through the AMPK Pathway
- PMID: 31434215
- PMCID: PMC6721273
- DOI: 10.3390/ijms20164054
Lipolytic Effects of 3-Iodothyronamine (T1AM) and a Novel Thyronamine-Like Analog SG-2 through the AMPK Pathway
Abstract
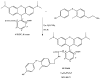
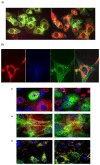
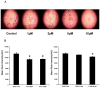
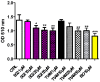
3-Iodothyronamine (T1AM) and its synthetic analog SG-2 are rapidly emerging as promising drivers of cellular metabolic reprogramming. Our recent research indicates that in obese mice a sub-chronic low dose T1AM treatment increased lipolysis, associated with significant weight loss independent of food consumption. The specific cellular mechanism of T1AM's lipolytic effect and its site of action remains unknown. First, to study the mechanism used by T1AM to gain entry into cells, we synthesized a fluoro-labeled version of T1AM (FL-T1AM) by conjugating it to rhodamine (TRITC) and analyzed its cellular uptake and localization in 3T3-L1 mouse adipocytes. Cell imaging using confocal microscopy revealed a rapid intercellular uptake of FL-T1AM into mitochondria without localization to the lipid droplet or nucleus of mature adipocytes. Treatment of 3T3-L1 adipocytes with T1AM and SG-2 resulted in decreased lipid accumulation, the latter showing a significantly higher potency than T1AM (10 µM vs. 20 µM, respectively). We further examined the effects of T1AM and SG-2 on liver HepG2 cells. A significant decrease in lipid accumulation was observed in HepG2 cells treated with T1AM or SG-2, due to increased lipolytic activity. This was confirmed by accumulation of glycerol in the culture media and through activation of the AMPK/ACC signaling pathways.
Keywords: 3-iodothyronamine (T1AM), thyroid hormone analogs; AMPK pathway; lipid metabolism; metabolic reprogramming; mitochondria; rhodamine (TRITC), cell imaging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The metabolic suppressor 3-iodothyronamine enhances lipolysis in 3T3-L1 adipocytes via activation of the adenosine monophosphate-activated protein kinase/forkhead box O1 signaling pathway.J Physiol Pharmacol. 2020 Jun;71(3). doi: 10.26402/jpp.2020.3.12. Epub 2020 Oct 15. J Physiol Pharmacol. 2020. PMID: 33077693
-
Endogenous 3-Iodothyronamine (T1AM) and Synthetic Thyronamine-like Analog SG-2 Act as Novel Pleiotropic Neuroprotective Agents Through the Modulation of SIRT6.Molecules. 2020 Feb 26;25(5):1054. doi: 10.3390/molecules25051054. Molecules. 2020. PMID: 32110992 Free PMC article.
-
Uptake of 3-iodothyronamine hormone analogs inhibits the growth and viability of cancer cells.FEBS Open Bio. 2017 Mar 6;7(4):587-601. doi: 10.1002/2211-5463.12205. eCollection 2017 Apr. FEBS Open Bio. 2017. PMID: 28396842 Free PMC article.
-
Central Effects of 3-Iodothyronamine Reveal a Novel Role for Mitochondrial Monoamine Oxidases.Front Endocrinol (Lausanne). 2018 Jun 6;9:290. doi: 10.3389/fendo.2018.00290. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29928258 Free PMC article. Review.
-
The 3-iodothyronamine (T1AM) and the 3-iodothyroacetic acid (TA1) indicate a novel connection with the histamine system for neuroprotection.Eur J Pharmacol. 2021 Dec 5;912:174606. doi: 10.1016/j.ejphar.2021.174606. Epub 2021 Oct 27. Eur J Pharmacol. 2021. PMID: 34717926 Review.
Cited by
-
Identification of a Thyroid Hormone Derivative as a Pleiotropic Agent for the Treatment of Alzheimer's Disease.Pharmaceuticals (Basel). 2021 Dec 19;14(12):1330. doi: 10.3390/ph14121330. Pharmaceuticals (Basel). 2021. PMID: 34959730 Free PMC article.
-
Thyroid hormone signaling in ocular development and diseases.Biol Res. 2025 Jul 2;58(1):42. doi: 10.1186/s40659-025-00618-1. Biol Res. 2025. PMID: 40605078 Free PMC article. Review.
-
Targeting autophagy impairment improves the phenotype of a novel CLN8 zebrafish model.Neurobiol Dis. 2024 Jul;197:106536. doi: 10.1016/j.nbd.2024.106536. Epub 2024 May 17. Neurobiol Dis. 2024. PMID: 38763444 Free PMC article.
-
Genetic advancements in obesity management and CRISPR-Cas9-based gene editing system.Mol Cell Biochem. 2023 Mar;478(3):491-501. doi: 10.1007/s11010-022-04518-w. Epub 2022 Jul 31. Mol Cell Biochem. 2023. PMID: 35909208 Review.
-
Delivery of Thyronamines (TAMs) to the Brain: A Preliminary Study.Molecules. 2021 Mar 14;26(6):1616. doi: 10.3390/molecules26061616. Molecules. 2021. PMID: 33799468 Free PMC article.
References
-
- Hoefig C.S., Kohrle J., Brabant G., Dixit K., Yap B., Strasburger C.J., Wu Z. Evidence for extrathyroidal formation of 3-iodothyronamine in humans as provided by a novel monoclonal antibody-based chemiluminescent serum immunoassay. J. Clin. Endocrinol. Metab. 2011;96:1864–1872. doi: 10.1210/jc.2010-2680. - DOI - PubMed
-
- Haviland J.A., Reiland H., Butz D.E., Tonelli M., Porter W.P., Zucchi R., Scanlan T.S., Chiellini G., Assadi-Porter F.M. Nmr-based metabolomics and breath studies show lipid and protein catabolism during low dose chronic t1 am treatment. Obesity. 2013;21:2538–2544. doi: 10.1002/oby.20391. - DOI - PMC - PubMed
-
- Panas H.N., Lynch L.J., Vallender E.J., Xie Z., Chen G.L., Lynn S.K., Scanlan T.S., Miller G.M. Normal thermoregulatory responses to 3-iodothyronamine, trace amines and amphetamine-like psychostimulants in trace amine associated receptor 1 knockout mice. J. Neurosci. Res. 2010;88:1962–1969. doi: 10.1002/jnr.22367. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

