Therapeutic and Protective Effects of Liposomal Encapsulation of Astaxanthin in Mice with Alcoholic Liver Fibrosis
- PMID: 31434227
- PMCID: PMC6718996
- DOI: 10.3390/ijms20164057
Therapeutic and Protective Effects of Liposomal Encapsulation of Astaxanthin in Mice with Alcoholic Liver Fibrosis
Abstract
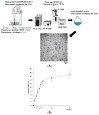
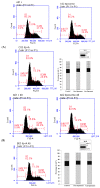
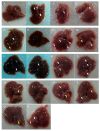
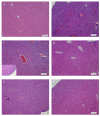
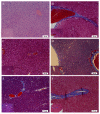
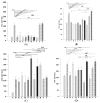
Astaxanthin (Asta) has been demonstrated to possess anti-inflammatory, antitumor, and free radical-clearing activities. However, the poor stability and low water solubility of Asta hamper its bioavailability. The objectives of this study were to fabricate Asta-loaded liposomes (Asta-lipo) and investigate the therapeutic effects of Asta-lipo on alcoholic liver fibrosis in mice. The mice were administered with Asta-lipo or liposomes alone prior to a 3-week dose containing 30% alcohol with or without feeding with a second dose of 30% alcohol. The prepared Asta-lipo of 225.0 ± 58.3 nm in diameter, had an encapsulation efficiency of 98%. A slow release profile of 16.2% Asta from Asta-lipo was observed after a 24-h incubation. Restorative actions against alcoholic liver fibrosis were observed after oral administration of Asta-lipo for 4 weeks. Hepatic repair, followed by a second dose of 30% alcohol, suggested that Asta-lipo exerted protective and reparative effects against liver injuries induced by repeated consumption of alcohol. The changes of serum ALT and AST values were principally in consistence with the histopathologic findings. Asta-lipo exerted rapid and direct effects against repeated alcohol-induced liver disease, whereas Asta-lipo given orally could boost recovery from liver injuries obtained due to previous long-term alcohol use. These data demonstrate that Asta-lipo has applicable protective and therapeutic potential to treat alcohol-induced liver diseases.
Keywords: alcohol; astaxanthin; intraperitoneal injection; liposome; liver fibrosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Natalia A.O., Srivatsan K. In vitro studies of alcohol-induced liver injury in virally-infected human hepatocytes: Advantages and limitations. Int. J. Hepatobiliary Pancreat. Dis. 2017;7:50–52.
-
- Le Marchand L., Hankin J.H., Kolonel L.N., Beecher G.R., Wilkens L.R., Zhao L.P. Intake of specific carotenoids and lung cancer risk. Cancer Epidemiol. Biomark. Prev. 1993;2:183–187. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

