Advances in Biodegradable Nano-Sized Polymer-Based Ocular Drug Delivery
- PMID: 31434273
- PMCID: PMC6722735
- DOI: 10.3390/polym11081371
Advances in Biodegradable Nano-Sized Polymer-Based Ocular Drug Delivery
Abstract
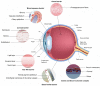
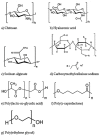
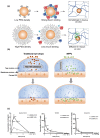
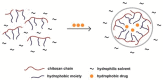
The effective delivery of drugs to the eye remains a challenge. The eye has a myriad of defense systems and physiological barriers that leaves ocular drug delivery systems with low bioavailability profiles. This is mainly due to poor permeability through the epithelia and rapid clearance from the eye following administration. However, recent advances in both polymeric drug delivery and biomedical nanotechnology have allowed for improvements to be made in the treatment of ocular conditions. The employment of biodegradable polymers in ocular formulations has led to improved retention time, greater bioavailability and controlled release through mucoadhesion to the epithelia in the eye, amongst other beneficial properties. Nanotechnology has been largely investigated for uses in the medical field, ranging from diagnosis of disease to treatment. The nanoscale of these developing drug delivery systems has helped to improve the penetration of drugs through the various ocular barriers, thus improving bioavailability. This review will highlight the physiological barriers encountered in the eye, current conventional treatment methods as well as how polymeric drug delivery and nanotechnology can be employed to optimize drug penetration to both the anterior and posterior segment of the eye.
Keywords: biodegradable; nanotechnology; ocular drug delivery; polymers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

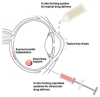
References
-
- Imam A.S.S., Bukhari A.S.N., Ahmad J., Ali A. Formulation and Optimization of Levofloxacin Loaded Chitosan Nanoparticle for Ocular Delivery: In Vitro Characterization, Ocular Tolerance and Antibacterial Activity. Int. J. Biol. Macromol. 2018;108:650–659. - PubMed
-
- Brown D., Heier J.S., Boyer D.S., Fruend K.B., Kaiser P., Kim J.E., Sarraf D. Current Best Clinical Practices–Management of Neovascular AMD. J. Vitreoretinal Dis. 2017;9:294–297. doi: 10.1177/2474126417725946. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

