Ground State Destabilization in Uracil DNA Glycosylase: Let's Not Forget "Tautomeric Strain" in Substrates
- PMID: 31434485
- PMCID: PMC6726543
- DOI: 10.1021/jacs.9b06447
Ground State Destabilization in Uracil DNA Glycosylase: Let's Not Forget "Tautomeric Strain" in Substrates
Abstract
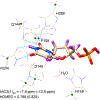
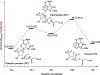
Enzymes like uracil DNA glycosylase (UDG) can achieve ground state destabilization, by polarizing substrates to mimic rare tautomers. On the basis of computed nucleus independent chemical shifts, NICS(1)zz, and harmonic oscillator model of electron delocalization (HOMED) analyses, of quantum mechanics (QM) and quantum mechanics/molecular mechanics (QM/MM) models of the UDG active site, uracil is strongly polarized when bound to UDG and resembles a tautomer >12 kcal/mol higher in energy. Natural resonance theory (NRT) analyses identified a dominant O2 imidate resonance form for residue bound 1-methyl-uracil. This "tautomeric strain" raises the energy of uracil, making uracilate a better than expected leaving group. Computed gas-phase SN2 reactions of free and hydrogen bonded 1-methyl-uracil demonstrate the relationship between the degree of polarization in uracil and the leaving group ability of uracilate.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Jencks WP Catalysis in Chemistry and Enzymology; McGraw-Hill: New York, 1969; p 282.
-
- Menger FM Analysis of ground-state and transition-state effects in enzyme catalysis. Biochemistry 1992, 31, 5368–5373. - PubMed
-
- Savva R; McAuley-Hecht K; Brown T; Pearl L The structural basis of specific base-excision repair by uracil–DNA glycosylase. Nature 1995, 373, 487–493. - PubMed
-
- Mol CD; Arvai AS; Slupphaug G; Kavli B; Alseth I; Krokan HE; Tainer JA Crystal structure of human uracil−DNA glycosylase in complex with a protein inhibitor: Protein mimicry of DNA. Cell 1995, 80, 869–878. - PubMed
-
- Slupphaug G; Mol CD; Kavli B; Arvai AS; Krokan HE; Tainer JA A nucleotide-flipping mechanism from the structure of human uracil–DNA glycosylase bound to DNA. Nature 1996, 384, 87–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

