Multidimensional Proteomics Identifies Declines in Protein Homeostasis and Mitochondria as Early Signals for Normal Aging and Age-associated Disease in Drosophila
- PMID: 31434710
- PMCID: PMC6773560
- DOI: 10.1074/mcp.RA119.001621
Multidimensional Proteomics Identifies Declines in Protein Homeostasis and Mitochondria as Early Signals for Normal Aging and Age-associated Disease in Drosophila
Abstract
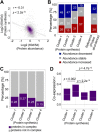
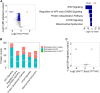
Aging is characterized by a gradual deterioration in proteome. However, how protein dynamics that changes with normal aging and in disease is less well understood. Here, we profiled the snapshots of aging proteome in Drosophila, from head and muscle tissues of post-mitotic somatic cells, and the testis of mitotically-active cells. Our data demonstrated that dysregulation of proteome homeostasis, or proteostasis, might be a common feature associated with age. We further used pulsed metabolic stable isotope labeling analysis to characterize protein synthesis. Interestingly, this study determined an age-modulated decline in protein synthesis with age, particularly in the pathways related to mitochondria, neurotransmission, and proteostasis. Importantly, this decline became dramatically accelerated in Pink1 mutants, a Drosophila model of human age-related Parkinson's disease. Taken together, our multidimensional proteomic study revealed tissue-specific protein dynamics with age, highlighting mitochondrial and proteostasis-related proteins. We suggest that declines in proteostasis and mitochondria early in life are critical signals prior to the onset of aging and aging-associated diseases.
Keywords: Drosophila melanogaster; aging; mitochondria function or biology; protein synthesis; proteome homeostasis; quantification.
© 2019 Yang et al.
Figures





References
-
- Kirkwood T. B. L., and Austad S. N. (2000) Why do we age? Nature 408, 233–238 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

