The HIV-1 Antisense Protein ASP Is a Transmembrane Protein of the Cell Surface and an Integral Protein of the Viral Envelope
- PMID: 31434734
- PMCID: PMC6803264
- DOI: 10.1128/JVI.00574-19
The HIV-1 Antisense Protein ASP Is a Transmembrane Protein of the Cell Surface and an Integral Protein of the Viral Envelope
Abstract
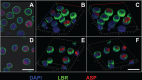
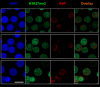
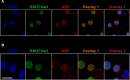
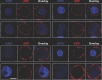
The negative strand of HIV-1 encodes a highly hydrophobic antisense protein (ASP) with no known homologs. The presence of humoral and cellular immune responses to ASP in HIV-1 patients indicates that ASP is expressed in vivo, but its role in HIV-1 replication remains unknown. We investigated ASP expression in multiple chronically infected myeloid and lymphoid cell lines using an anti-ASP monoclonal antibody (324.6) in combination with flow cytometry and microscopy approaches. At baseline and in the absence of stimuli, ASP shows polarized subnuclear distribution, preferentially in areas with low content of suppressive epigenetic marks. However, following treatment with phorbol 12-myristate 13-acetate (PMA), ASP translocates to the cytoplasm and is detectable on the cell surface, even in the absence of membrane permeabilization, indicating that 324.6 recognizes an ASP epitope that is exposed extracellularly. Further, surface staining with 324.6 and anti-gp120 antibodies showed that ASP and gp120 colocalize, suggesting that ASP might become incorporated in the membranes of budding virions. Indeed, fluorescence correlation spectroscopy studies showed binding of 324.6 to cell-free HIV-1 particles. Moreover, 324.6 was able to capture and retain HIV-1 virions with efficiency similar to that of the anti-gp120 antibody VRC01. Our studies indicate that ASP is an integral protein of the plasma membranes of chronically infected cells stimulated with PMA, and upon viral budding, ASP becomes a structural protein of the HIV-1 envelope. These results may provide leads to investigate the possible role of ASP in the virus replication cycle and suggest that ASP may represent a new therapeutic or vaccine target.IMPORTANCE The HIV-1 genome contains a gene expressed in the opposite, or antisense, direction to all other genes. The protein product of this antisense gene, called ASP, is poorly characterized, and its role in viral replication remains unknown. We provide evidence that the antisense protein, ASP, of HIV-1 is found within the cell nucleus in unstimulated cells. In addition, we show that after PMA treatment, ASP exits the nucleus and localizes on the cell membrane. Moreover, we demonstrate that ASP is present on the surfaces of viral particles. Altogether, our studies identify ASP as a new structural component of HIV-1 and show that ASP is an accessory protein that promotes viral replication. The presence of ASP on the surfaces of both infected cells and viral particles might be exploited therapeutically.
Keywords: HIV-1; antisense protein ASP; cell surface protein; viral envelope protein.
Copyright © 2019 American Society for Microbiology.
Figures







References
-
- Gaudray G, Gachon F, Basbous J, Biard-Piechaczyk M, Devaux C, Mesnard JM. 2002. The complementary strand of the human T-cell leukemia virus type 1 RNA genome encodes a bZIP transcription factor that down-regulates viral transcription. J Virol 76:12813–12822. doi:10.1128/jvi.76.24.12813-12822.2002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

