BTLA suppress acute rejection via regulating TCR downstream signals and cytokines production in kidney transplantation and prolonged allografts survival
- PMID: 31434927
- PMCID: PMC6704067
- DOI: 10.1038/s41598-019-48520-7
BTLA suppress acute rejection via regulating TCR downstream signals and cytokines production in kidney transplantation and prolonged allografts survival
Erratum in
-
Author Correction: BTLA suppress acute rejection via regulating TCR downstream signals and cytokines production in kidney transplantation and prolonged allografts survival.Sci Rep. 2022 Dec 7;12(1):21175. doi: 10.1038/s41598-022-24559-x. Sci Rep. 2022. PMID: 36476626 Free PMC article. No abstract available.
Abstract
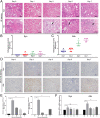
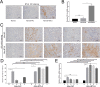
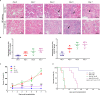
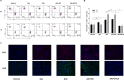
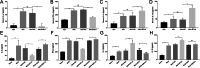
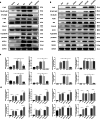
Acute rejection is a major risk for renal transplant failure. During this adverse process, activated T cells are considered the main effectors. Recently, B and T lymphocyte attenuator (BTLA), a member of the CD28 family receptor, was reported to be a novel inhibitory regulator of T cell activation in heart and pancreatic allograft rejection. Due to the similarity of acute rejection pathways among different organs, we hypothesized that BTLA might play a role in acute rejection of kidney transplant. In renal transplant patients, we observed that BTLA expression was significantly decreased in peripheral CD3+ T lymphocytes of biopsy-proven acute rejection (BPAR) recipients compared with control patients with stable transplanted kidney functions. Remarkably, overexpression of BTLA in the rat model was found to significantly inhibit the process of acute rejection, regulate the postoperative immune status, and prolong allograft survival. BTLA overexpression significantly suppressed IL-2 and IFN-γ production and increased IL-4 and IL-10 production both in vivo and in vitro. Moreover, vital factors in T-cell signaling pathways, including mitogen-associated protein kinases (MAPK), nuclear factor-kappa B (NF-κB) and nuclear factor of activated T cells (NFAT), were also significantly repressed by BTLA overexpression. Therefore, BTLA can suppress acute rejection and regulate allogeneic responses of kidney transplant by regulating TCR downstream signals and inflammatory cytokines production to improve allografts outcomes.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

