Identification of an ATP-sensitive potassium channel in mitochondria
- PMID: 31435016
- PMCID: PMC6726485
- DOI: 10.1038/s41586-019-1498-3
Identification of an ATP-sensitive potassium channel in mitochondria
Abstract
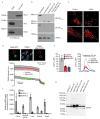
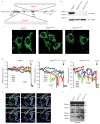
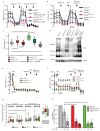
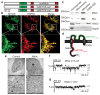
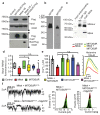
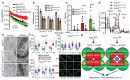
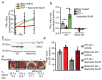
Mitochondria provide chemical energy for endoergonic reactions in the form of ATP, and their activity must meet cellular energy requirements, but the mechanisms that link organelle performance to ATP levels are poorly understood. Here we confirm the existence of a protein complex localized in mitochondria that mediates ATP-dependent potassium currents (that is, mitoKATP). We show that-similar to their plasma membrane counterparts-mitoKATP channels are composed of pore-forming and ATP-binding subunits, which we term MITOK and MITOSUR, respectively. In vitro reconstitution of MITOK together with MITOSUR recapitulates the main properties of mitoKATP. Overexpression of MITOK triggers marked organelle swelling, whereas the genetic ablation of this subunit causes instability in the mitochondrial membrane potential, widening of the intracristal space and decreased oxidative phosphorylation. In a mouse model, the loss of MITOK suppresses the cardioprotection that is elicited by pharmacological preconditioning induced by diazoxide. Our results indicate that mitoKATP channels respond to the cellular energetic status by regulating organelle volume and function, and thereby have a key role in mitochondrial physiology and potential effects on several pathological processes.
Conflict of interest statement
All authors declare no competing interests.
Figures






References
-
- Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–8. - PubMed
-
- Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–6. - PubMed
-
- Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244–247. - PubMed
-
- Paucek P, et al. Reconstitution and partial purification of the glibenclamide-sensitive, ATP-dependent K+ channel from rat liver and beef heart mitochondria. J Biol Chem. 1992;267:26062–26069. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

