Synaptic clustering differences due to different GABRB3 mutations cause variable epilepsy syndromes
- PMID: 31435640
- PMCID: PMC6776116
- DOI: 10.1093/brain/awz250
Synaptic clustering differences due to different GABRB3 mutations cause variable epilepsy syndromes
Abstract
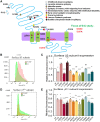
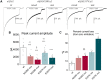
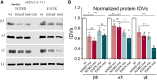
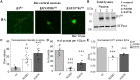
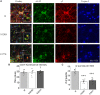
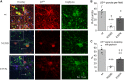
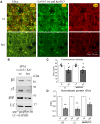
GABRB3 is highly expressed early in the developing brain, and its encoded β3 subunit is critical for GABAA receptor assembly and trafficking as well as stem cell differentiation in embryonic brain. To date, over 400 mutations or variants have been identified in GABRB3. Mutations in GABRB3 have been increasingly recognized as a major cause for severe paediatric epilepsy syndromes such as Lennox-Gastaut syndrome, Dravet syndrome and infantile spasms with intellectual disability as well as relatively mild epilepsy syndromes such as childhood absence epilepsy. There is no plausible molecular pathology for disease phenotypic heterogeneity. Here we used a very high-throughput flow cytometry assay to evaluate the impact of multiple human mutations in GABRB3 on receptor trafficking. In this study we found that surface expression of mutant β3 subunits is variable. However, it was consistent that surface expression of partnering γ2 subunits was lower when co-expressed with mutant than with wild-type subunits. Because γ2 subunits are critical for synaptic GABAA receptor clustering, this provides an important clue for understanding the pathophysiology of GABRB3 mutations. To validate our findings further, we obtained an in-depth comparison of two novel mutations [GABRB3 (N328D) and GABRB3 (E357K)] associated with epilepsy with different severities of epilepsy phenotype. GABRB3 (N328D) is associated with the relatively severe Lennox-Gastaut syndrome, and GABRB3 (E357K) is associated with the relatively mild juvenile absence epilepsy syndrome. With functional characterizations in both heterologous cells and rodent cortical neurons by patch-clamp recordings, confocal microscopy and immunoblotting, we found that both the GABRB3 (N328D) and GABRB3 (E357K) mutations reduced total subunit expression in neurons but not in HEK293T cells. Both mutant subunits, however, were reduced on the cell surface and in synapses, but the Lennox-Gastaut syndrome mutant β3 (N328D) subunit was more reduced than the juvenile absence epilepsy mutant β3 (E357K) subunit. Interestingly, both mutant β3 subunits impaired postsynaptic clustering of wild-type GABAA receptor γ2 subunits and prevented γ2 subunits from incorporating into GABAA receptors at synapses, although by different cellular mechanisms. Importantly, wild-type γ2 subunits were reduced and less clustered at inhibitory synapses in Gabrb3+/- knockout mice. This suggests that impaired receptor localization to synapses is a common pathophysiological mechanism for GABRB3 mutations, although the extent of impairment may be different among mutant subunits. The study thus identifies the novel mechanism of impaired targeting of receptors containing mutant β3 subunits and provides critical insights into understanding how GABRB3 mutations produce severe epilepsy syndromes and epilepsy phenotypic heterogeneity.
Keywords: GABRB3 mutation; GABAA receptor; Lennox-Gastaut syndrome; intellectual disability; juvenile absence epilepsy.
© The Author(s) (2019). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Andäng M, Hjerling-Leffler J, Moliner A, Lundgren TK, Castelo-Branco G, Nanou E et al. . Histone H2AX-dependent GABA(A) receptor regulation of stem cell proliferation. Nature 2008; 451: 460–64. - PubMed
-
- Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, Prud’homme JF et al. . First genetic evidence of GABA(A) receptor dysfunction in epilepsy: a mutation in the gamma2-subunit gene. Nat Genet 2001; 28: 46–8. - PubMed
-
- Brooks-Kayal A. Epilepsy and autism spectrum disorders: are there common developmental mechanisms? Brain Dev 2010; 32: 731–8. - PubMed

