Invasive left ventricle pressure-volume analysis: overview and practical clinical implications
- PMID: 31435675
- PMCID: PMC7084193
- DOI: 10.1093/eurheartj/ehz552
Invasive left ventricle pressure-volume analysis: overview and practical clinical implications
Abstract
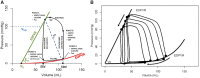
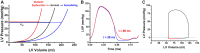
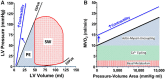
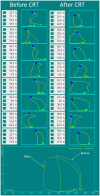
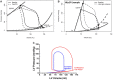
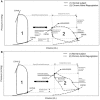
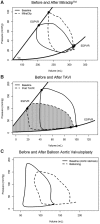
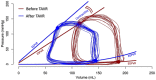
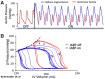
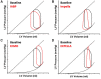
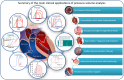
Ventricular pressure-volume (PV) analysis is the reference method for the study of cardiac mechanics. Advances in calibration algorithms and measuring techniques brought new perspectives for its application in different research and clinical settings. Simultaneous PV measurement in the heart chambers offers unique insights into mechanical cardiac efficiency. Beat to beat invasive PV monitoring can be instrumental in the understanding and management of heart failure, valvular heart disease, and mechanical cardiac support. This review focuses on intra cardiac left ventricular PV analysis principles, interpretation of signals, and potential clinical applications.
Keywords: Left ventricular haemodynamics; Myocardial energetics; Pressure-volume loop.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures



References
-
- Kuhtz-Buschbeck JP, Drake-Holland A, Noble MIM, Lohff B, Schaefer J.. Rediscovery of Otto Frank's contribution to science. J Mol Cell Cardiol 2018;119:96–103. - PubMed
-
- Suga H. Ventricular energetics. Physiol Rev 1990;70:247–277. - PubMed
-
- Baan J, van der Velde ET, Steendijk P.. Ventricular pressure-volume relations in vivo. Eur Heart J 1992;13 Suppl E:2–6. - PubMed
-
- van der Velde ET, van Dijk AD, Steendijk P, Diethelm L, Chagas T, Lipton MJ, Glanz SA, Baan J.. Left ventricular segmental volume by conductance catheter and Cine-CT. Eur Heart J 1992;13 Suppl E:15–21. - PubMed
-
- Steendijk P, Tulner SA, Schreuder JJ, Bax JJ, van Erven L, van der Wall EE, Dion RA, Schalij MJ, Baan J.. Quantification of left ventricular mechanical dyssynchrony by conductance catheter in heart failure patients. Am J Physiol Heart Circ Physiol 2004;286:H723–30. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

