Phenotypes of Chronic Hepatitis B in Children From a Large North American Cohort
- PMID: 31436702
- PMCID: PMC6814562
- DOI: 10.1097/MPG.0000000000002467
Phenotypes of Chronic Hepatitis B in Children From a Large North American Cohort
Abstract
Objective: The aim of the study was to define chronic HBV phenotypes in a large, cohort of United States and Canadian children utilizing recently published population-based upper limit of normal alanine aminotransferase levels (ULN ALT), compared with local laboratory ULN; identify relationships with host and viral factors.
Background: Chronic hepatitis B virus (HBV) infection has been characterized by phases or phenotypes, possibly associated with prognosis and indications for therapy.
Methods: Baseline enrollment data of children in the Hepatitis B Research Network were examined. Phenotype definitions were inactive carrier: HBeAg-negative with low HBV DNA and normal ALT levels; immune-tolerant: HBeAg-positive with high HBV DNA but normal ALT levels; or chronic hepatitis B: HBeAg-positive or -negative with high HBV DNA and abnormal ALT levels.
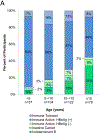
Results: Three hundred seventy-one participants were analyzed of whom 274 were HBeAg-positive (74%). Younger participants were more likely be HBeAg-positive with higher HBV DNA levels. If local laboratory ULN ALT levels were used, 35% were assigned the immune tolerant phenotype, but if updated ULN were applied, only 12% could be so defined, and the remaining 82% would be considered to have chronic hepatitis B. Among HBeAg-negative participants, only 21 (22%) were defined as inactive carriers and 14 (14%) as HBeAg-negative chronic hepatitis B; the majority (61%) had abnormal ALT and low levels of HBV DNA, thus having an indeterminant phenotype. Increasing age was associated with smaller proportions of HBeAg-positive infection.
Conclusions: Among children with chronic HBV infection living in North America, the immune tolerant phenotype is uncommon and HBeAg positivity decreases with age.
Conflict of interest statement
Disclosures:
KB Schwarz has research grants from Gilead, Bristol-Myers Squibb, Roche/Genentech and consults for Gilead, Roche/Genentech and Up to Date
Adrian M Di Bisceglie has research grants from Gilead and serves on advisory boards to Gilead and Bristol-Myers Squibb.
Karen F Murray has research grants supported by Gilead, Merck, Shire, and owns stock in Merck
Philip Rosenthal has research grants from Abbvie, Gilead, Bristol-Myers Squibb, Roche/Genentech and consults for Gilead, Abbvie, Intercept, Alexion, Retrophil, Albireo, Audentes and Mirum.
Simon C Ling receives research funding from Abbvie and Bristol-Myers Squibb.
Norberto Rodriguez-Baez has research grants supported by Gilead.
Jeffrey Teckman has research grants from Gilead, Alnylam Inc., Arrowhead Pharmaceuticals and Dicerna Inc., and consultant relationships with BioMarin, Editas, Proteostasis and Retrophin.
Yona Keich Cloonan, Manuel Lombardero, Sarah Jane Schwarzenberg and Jay H Hoofnagle have no duality or conflicts of interest.
Figures




References
-
- Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–2. - PubMed
-
- Tong MJ, Trieu J. Hepatitis B inactive carriers: clinical course and outcomes. J Dig Dis. 2013;14:311–7. - PubMed
-
- Wirth S, Bortolotti F, Brunert C, et al. Hepatitis B virus genotype change in children is closely related to HBeAg/anti-HBe seroconversion. J Pediatr Gastroenterol Nutr. 2013;57:363–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

