Association of a Primary Open-Angle Glaucoma Genetic Risk Score With Earlier Age at Diagnosis
- PMID: 31436842
- PMCID: PMC6707005
- DOI: 10.1001/jamaophthalmol.2019.3109
Association of a Primary Open-Angle Glaucoma Genetic Risk Score With Earlier Age at Diagnosis
Abstract
Importance: Genetic variants associated with primary open-angle glaucoma (POAG) are known to influence disease risk. However, the clinical effect of associated variants individually or in aggregate is not known. Genetic risk scores (GRS) examine the cumulative genetic load by combining individual genetic variants into a single measure, which is assumed to have a larger effect and increased power to detect relevant disease-related associations.
Objective: To investigate if a GRS that comprised 12 POAG genetic risk variants is associated with age at disease diagnosis.
Design, setting, and participants: A cross-sectional study included individuals with POAG and controls from the Glaucoma Genes and Environment (GLAUGEN) study and the National Eye Institute Glaucoma Human Genetics Collaboration (NEIGHBOR) study. A GRS was formulated using 12 variants known to be associated with POAG, and the alleles associated with increasing risk of POAG were aligned in the case-control sets. In case-only analyses, the association of the GRS with age at diagnosis was analyzed as an estimate of disease onset. Results from cohort-specific analyses were combined with meta-analysis. Data collection started in August 2012 for the NEIGHBOR cohort and in July 2008 for the GLAUGEN cohort and were analyzed starting in March 2018.
Main outcomes and measures: Association of a 12 single-nucleotide polymorphism POAG GRS with age at diagnosis in individuals with POAG using linear regression.
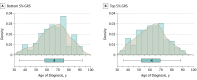
Results: The GLAUGEN study included 976 individuals with POAG and 1140 controls. The NEIGHBOR study included 2132 individuals with POAG and 2290 controls. For individuals with POAG, the mean (SD) age at diagnosis was 63.6 (9.8) years in the GLAUGEN cohort and 66.0 (13.7) years in the NEIGHBOR cohort. For controls, the mean (SD) age at enrollment was 65.5 (9.2) years in the GLAUGEN cohort and 68.9 (11.4) years in the NEIGHBOR cohort. All study participants were European white. The GRS was strongly associated with POAG risk in case-control analysis (odds ratio per 1-point increase in score = 1.24; 95% CI, 1.21-1.27; P = 3.4 × 10-66). In case-only analyses, each higher GRS unit was associated with a 0.36-year earlier age at diagnosis (β = -0.36; 95% CI, -0.56 to -0.16; P = 4.0 × 10-4). Individuals in the top 5% of the GRS had a mean (SD) age at diagnosis of 5.2 (12.8) years earlier than those in the bottom 5% GRS (61.4 [12.7] vs 66.6 [12.9] years; P = 5.0 × 10-4).
Conclusions and relevance: A higher dose of POAG risk alleles was associated with an earlier age at glaucoma diagnosis. On average, individuals with POAG with the highest GRS had 5.2-year earlier age at diagnosis of disease. These results suggest that a GRS that comprised genetic variants associated with POAG could help identify patients with risk of earlier disease onset impacting screening and therapeutic strategies.
Conflict of interest statement
Figures