Airway epithelium-shifted mast cell infiltration regulates asthmatic inflammation via IL-33 signaling
- PMID: 31437129
- PMCID: PMC6819127
- DOI: 10.1172/JCI126402
Airway epithelium-shifted mast cell infiltration regulates asthmatic inflammation via IL-33 signaling
Abstract
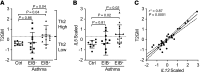
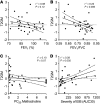
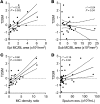
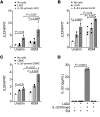
Asthma is a heterogeneous syndrome that has been subdivided into physiologic phenotypes and molecular endotypes. The most specific phenotypic manifestation of asthma is indirect airway hyperresponsiveness (AHR), and a prominent molecular endotype is the presence of type 2 inflammation. The underlying basis for type 2 inflammation and its relationship to AHR are incompletely understood. We assessed the expression of type 2 cytokines in the airways of subjects with and without asthma who were extensively characterized for AHR. Using quantitative morphometry of the airway wall, we identified a shift in mast cells from the submucosa to the airway epithelium specifically associated with both type 2 inflammation and indirect AHR. Using ex vivo modeling of primary airway epithelial cells in organotypic coculture with mast cells, we show that epithelial-derived IL-33 uniquely induced type 2 cytokines in mast cells, which regulated the expression of epithelial IL33 in a feed-forward loop. This feed-forward loop was accentuated in epithelial cells derived from subjects with asthma. These results demonstrate that type 2 inflammation and indirect AHR in asthma are related to a shift in mast cell infiltration to the airway epithelium, and that mast cells cooperate with epithelial cells through IL-33 signaling to regulate type 2 inflammation.
Keywords: Asthma; Immunology; Mast cells; Pulmonology; Th2 response.
Conflict of interest statement
Figures





References
-
- Martinez FD. Links between pediatric and adult asthma. J Allergy Clin Immunol. 2001;107(5 suppl):S449–S455. - PubMed

