Characterization and analysis of estrogenic cyclic phenone metabolites produced in vitro by rainbow trout liver slices using GC-MS, LC-MS and LC-TOF-MS
- PMID: 31437775
- PMCID: PMC6779324
- DOI: 10.1016/j.jchromb.2019.121717
Characterization and analysis of estrogenic cyclic phenone metabolites produced in vitro by rainbow trout liver slices using GC-MS, LC-MS and LC-TOF-MS
Abstract
Cyclic phenones are chemicals of interest to the USEPA and international organizations due to their potential for endocrine disruption to aquatic and terrestrial species. The metabolic conversion of cyclic phenones by liver hepatocytes and the structure of main metabolites yielded have not been assessed in fish species. As part of a larger project, in this study we investigated the structure of metabolites produced in vitro by rainbow trout (rt) liver slices after exposure to the model cyclic phenones benzophenone (DPK), cyclobutyl phenyl ketone (CBP) and cyclohexyl phenyl ketone (CPK). While only one distinct metabolite was detected for DPK and CBP (benzhydrol and CBPOH, respectively), CPK yielded nine positional isomers (M1-M9) as products. In absence of standards, improved inference of CPK metabolites tentative structures was achieved by combining GC-MS with and without derivatization, LC with tandem MS, LC with high resolution time of flight (TOF) MS and LC fractionation data with CPK phase II conjugative metabolism information. Data supported that CPK is metabolized by phase I oxidation of the cyclohexyl ring and not the phenyl group as predicted by metabolism simulators. CPK metabolites M1 and M2 (MW 186), were proposed to be cyclohexenyl-derivatives. Also, M6-M9 were proposed to be hydroxylated metabolites (MW 204), with the potential for undergoing phase II conjugative metabolism to glucuronides and sulfates. Finally, M3, M4 and M5 were proposed as cyclohexanone-derivatives of CPK (MW 202), resulting from the limited redox-interconversion of their hydroxylated pairs M8, M6 and M7, respectively. Assessment of metabolite role in biological responses associated with endocrine disruption will advance the development of methods for species extrapolation and the understanding of differential sensitivity of species to chemical exposure.
Keywords: Conjugative metabolism; Endocrine disruption; Fish; In vitro; Mass spectrometry techniques; Tentative metabolite structure.
Published by Elsevier B.V.
Conflict of interest statement
Declaration of Interest
All studies reported in this manuscript were supported by the USEPA, Office of Research and Development (ORD) and conducted by or under the supervision of USEPA employees in additional non-financial collaboration with the University of North Dakota. Manuscript review was performed in accordance with guidelines of the USEPA-ORD. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. Ideas discussed in the text are those of the authors and not necessarily the opinion of the USEPA. The authors report no conflict of interest.
Figures
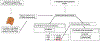
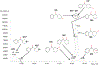

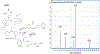
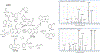



References
-
- Ankley G, Schmieder P., Serrano J, et al. Adverse outcomes pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem, 2010, 29:730–741. - PubMed
-
- Hornung M, Tapper M, Denny J, et al. Effects-based chemical category approach for prioritization of low affinity estrogenic chemicals. SAR QSAR Environ. Res, 2014, 25:132–154. - PubMed
-
- Hu G, Siu S, Li S, et al. Metabolism of calycosin, an isoflavone from Astragali Radix, in zebrafish larvae. Xenobiotica, 2012, 42(3):294–303. - PubMed
-
- Salinas K, Serrano J, Higgins L, et al. Identification of estrogen-responsive vitelline envelope protein fragments from Rainbow trout plasma using Mass Spectrometry. Mol. Reprod. Dev, 2010, 77(11):963–969. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

