The innovation of the final moult and the origin of insect metamorphosis
- PMID: 31438822
- PMCID: PMC6711288
- DOI: 10.1098/rstb.2018.0415
The innovation of the final moult and the origin of insect metamorphosis
Abstract
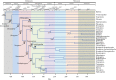
The three modes of insect postembryonic development are ametaboly, hemimetaboly and holometaboly, the latter being considered the only significant metamorphosis mode. However, the emergence of hemimetaboly, with the genuine innovation of the final moult, represents the origin of insect metamorphosis and a necessary step in the evolution of holometaboly. Hemimetaboly derives from ametaboly and might have appeared as a consequence of wing emergence in Pterygota, in the early Devonian. In extant insects, the final moult is mainly achieved through the degeneration of the prothoracic gland (PG), after the formation of the winged and reproductively competent adult stage. Metamorphosis, including the formation of the mature wings and the degeneration of the PG, is regulated by the MEKRE93 pathway, through which juvenile hormone precludes the adult morphogenesis by repressing the expression of transcription factor E93, which triggers this change. The MEKRE93 pathway appears conserved in extant metamorphosing insects, which suggest that this pathway was operative in the Pterygota last common ancestor. We propose that the final moult, and the consequent hemimetabolan metamorphosis, is a monophyletic innovation and that the role of E93 as a promoter of wing formation and the degeneration of the PG was mechanistically crucial for their emergence. This article is part of the theme issue 'The evolution of complete metamorphosis'.
Keywords: E93; evolution of insect metamorphosis; juvenile hormone; mekre93 pathway; origin of insect final moult; prothoracic gland degeneration.
Conflict of interest statement
I declare I have no competing interests.
Figures




References
-
- Sehnal F, Svacha P, Zrzavy J. 1996. Evolution of insect metamorphosis. In Metamorphosis. Postembryonic reprogramming of gene expression in amphibian and insect cells (eds Gilbert LI, Tata JR, Atkinson BG), pp. 3–58. San Diego, CA: Academic Press.
-
- Belles X. 2011. Origin and evolution of insect metamorphosis. In Encyclopedia of life sciences (ELS), pp. 1–11. Chichester, UK: John Wiley & Sons, Ltd; (10.1002/9780470015902.a0022854) - DOI
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
