Relationship between alterations of urinary microbiota and cultured negative lower urinary tract symptoms in female type 2 diabetes patients
- PMID: 31438919
- PMCID: PMC6704724
- DOI: 10.1186/s12894-019-0506-0
Relationship between alterations of urinary microbiota and cultured negative lower urinary tract symptoms in female type 2 diabetes patients
Abstract
Background: Lower urinary tract symptoms (LUTS) is the most common complication of diabetes. However, the underlying pathogenesis of cultured negative LUTS (cn-LUTS) in diabetic patients has not been well understood. Numerous evidence indicates that urinary dysbiosis is related to urologic disorders. We aim to study alterations of the urinary microbiota of cn-LUTS in type 2 diabetes (T2D) patients.
Methods: Female T2D patients and controls were recruited and requested to finish the American Urological Association Symptom Index. Mid-stream urine was collected for culturing and extracting DNA. Microbial diversity and composition were analyzed by targeting to 16S rDNA. Linear discriminant analysis effect size (LEfSe) was carried out to identify significantly different bacteria.
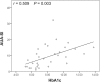
Results: 32 female T2D patients and 26 controls were enrolled. No significant differences in alpha diversity were observed between patients and controls. However, statistically decreased richness (ACE index and Chao 1 index, 85.52(13.75, 204.84) vs. 129.82(63.89, 280.30) and 83.86(11.00, 210.77) vs. 125.19(62.00, 251.77), P = 0.005; Observed Species, 76(10, 175) vs. 98(54, 234), P = 0.011) and decreased species diversity (Shannon index, 1.37(0.04, 3.48) vs. 2.09(0.98, 3.43), P = 0.033; Simpson index, 0.46 (0.06, 0.99) vs. 0.23(0.07, 0.64), P = 0.029) were shown in moderate-to-severe LUTS group and high Hemoglobin A1c group, respectively. A significant difference of beta diversity was found between T2D patients and controls and T2D patients with different severity of cn-LUTS as well as the different level of Hemoglobin A1c. LEfSe revealed that 10 genera (e.g., Escherichia-Shigella and Klebsiella) were increased and 7 genera were decreasing in T2D patients, 3 genera (e.g., Escherichia-Shigella and Campylobacter) were increased and 16 genera (e.g., Prevotella) were reduced in moderate-to-severe LUTS group, 2 genera (Escherichia-Shigella and Lactobacillus) were over-represented and 10 genera (e.g., Prevotella) were under-represented in high Hemoglobin A1c group. Finally, Hemoglobin A1c was found positively correlated with the total score of the American Urological Association Symptom Index (r = 0.509, P = 0.003).
Conclusions: Urinary dysbiosis may be related to cn-LUTS in female T2D patients. A better understanding of urinary microbiota in the development and progression of cn-LUTS in female T2D patients was necessary. The severity of cn-LUTS was correlated to hyperglycemia and chronic hyperglycemia might induce or promote cn-LUTS by influencing urinary microbiota.
Keywords: Diabetes mellitus; Hemoglobin A1c; Lower urinary tract symptoms; Urinary microbiota.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

