Tumor-derived exosomes, myeloid-derived suppressor cells, and tumor microenvironment
- PMID: 31438991
- PMCID: PMC6704713
- DOI: 10.1186/s13045-019-0772-z
Tumor-derived exosomes, myeloid-derived suppressor cells, and tumor microenvironment
Abstract
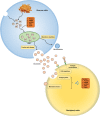
Plenty of immune cells infiltrate into the tumor microenvironment (TME) during tumor progression, in which myeloid-derived suppressor cells (MDSCs) represent a heterogeneous population of immature myeloid cells with immunosuppressive activity. Tumor cells and stromal cells facilitate the activation and expansion of MDSCs in TME via intercellular communication, and expanded MDSCs suppress anti-tumor immune responses through direct and indirect mechanisms. Currently, exosomes, which are a kind of extracellular vesicles (EVs) that can convey functional components, are demonstrated to participate in the local and distal intercellular communication between cells. Numerous studies have supposed that tumor-derived exosomes (TEXs), whose assembly and release can be modulated by TME, are capable of modulating the cell biology of MDSCs, including facilitating their activation, promoting the expansion, and enhancing the immunosuppressive function. Therefore, in this review, we mainly focus on the role of TEXs in the cell-cell communication between tumor cells and MDSCs, and discuss their clinical applications.
Keywords: Immunosuppression; Intercellular communication; Myeloid-derived suppressor cells; Tumor microenvironment; Tumor-derived exosomes.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, et al. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 2006;66(18):9290–9298. doi: 10.1158/0008-5472.CAN-06-1819. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

