Role of immune checkpoint inhibitor-based therapies for metastatic renal cell carcinoma in the first-line setting: A Bayesian network analysis
- PMID: 31439476
- PMCID: PMC6796578
- DOI: 10.1016/j.ebiom.2019.08.006
Role of immune checkpoint inhibitor-based therapies for metastatic renal cell carcinoma in the first-line setting: A Bayesian network analysis
Abstract
Background: Several novel immune checkpoint inhibitor (ICI)-based treatments exhibited promising survival benefits for metastatic renal cell carcinoma (mRCC), yet there is no current guidance regarding the optimum first-line regimen. We performed this network analysis to compare the efficacy and safety of all available treatments for mRCC.
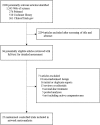
Methods: A systematic search of literature was conducted up to April 30, 2019, and the analysis was done on a Bayesian fixed-effect model.
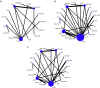
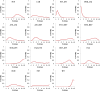
Findings: Twenty-five randomized clinical trials (RCTs) involving 13,010 patients were included in this study. The results showed that for overall survival, pembrolizumab plus axitinib (hazard ratio [HR]: 0.53; 95% credible interval [CrI]: 0.38-0.73) and nivolumab plus ipilimumab (HR: 0.63; 95% CrI: 0.50-0.79) were significantly more effective than sunitinib, and pembrolizumab plus axitinib was probably (68%) to be the best choice. For progression-free survival, cabozantinib (HR: 0.66; 95% CrI: 0.46-0.94), pembrolizumab plus axitinib (HR: 0.69; 95% CrI: 0.57-0.84), avelumab plus axitinib (HR: 0.69; 95% CrI: 0.56-0.85), nivolumab plus ipilimumab (HR: 0.82; 95% CrI: 0.68-0.99), and atezolizumab plus bevacizumab (HR: 0.86; 95% CrI: 0.74-0.99) were statistically superior to sunitinib, and cabozantinib was likely (43%) to be the preferred options. Nivolumab plus ipilimumab (OR: 0.50; 95% CrI: 0.28-0.84), and atezolizumab plus bevacizumab (OR: 0.56; 95% CrI: 0.36-0.83) were associated with significantly lower rate of high-grade adverse events than sunitinib.
Interpretation: Our findings demonstrate that pembrolizumab plus axitinib might be the best treatment for mRCC, while nivolumab plus ipilimumab has the most favorable balance between efficacy and acceptability, and may provide new guidance to make treatment decisions. FUND: This research was supported by the Henan Provincial Scientific and Technological Research Project (Grant No. 192102310036).
Keywords: Efficacy; First-line systemic therapies; Immune checkpoint inhibitor; Renal cell carcinoma; Safety.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
None.
Figures



Comment in
-
Management of metastatic renal cell carcinoma: The complexity of choice.EBioMedicine. 2019 Sep;47:2-3. doi: 10.1016/j.ebiom.2019.08.049. Epub 2019 Sep 6. EBioMedicine. 2019. PMID: 31501075 Free PMC article. No abstract available.
References
-
- Stitzlein L., Rao P., Dudley R. Emerging oral VEGF inhibitors for the treatment of renal cell carcinoma. Expert Opin Investig Drugs. 2019;28(2):121–130. - PubMed
-
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. - PubMed
-
- Posadas E.M., Limvorasak S., Figlin R.A. Targeted therapies for renal cell carcinoma. Nat Rev Nephrol. 2017;13(8):496–511. - PubMed
-
- Considine B., Hurwitz M.E. Current status and future directions of immunotherapy in renal cell carcinoma. Curr Oncol Rep. 2019;21(4):34. - PubMed
-
- Fyfe G., Fisher R.I., Rosenberg S.A., Sznol M., Parkinson D.R., Louie A.C. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13(3):688–696. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

