Phase I Trial of Well-Differentiated Neuroendocrine Tumors (NETs) with Radiolabeled Somatostatin Antagonist 177Lu-Satoreotide Tetraxetan
- PMID: 31439583
- PMCID: PMC8382090
- DOI: 10.1158/1078-0432.CCR-19-1026
Phase I Trial of Well-Differentiated Neuroendocrine Tumors (NETs) with Radiolabeled Somatostatin Antagonist 177Lu-Satoreotide Tetraxetan
Abstract
Purpose: Radiolabeled somatostatin receptor 2 (SSTR2) antagonists have shown higher tumor uptake and tumor-to-organ ratios than somatostatin agonists in preclinical models of neuroendocrine tumors (NETs). We performed a phase I study to evaluate the safety and efficacy of SSTR2 antagonist 177Lu-satoreotide tetraxetan.
Patients and methods: Twenty patients with advanced SSTR2-positive NETs were treated with 177Lu-satoreotide tetraxetan. Patients first underwent a dosimetry study with 177Lu-satoreotide tetraxetan to determine the therapeutic activity that could be safely administered. This activity was split into two equal cycles to be delivered 3 months apart. The maximum activity was 7.4 GBq per cycle.
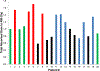
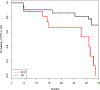
Results: Of 20 patients with NETs (one lung, seven small bowel, nine pancreatic, one gastric, one rectal, one kidney; mean prior treatments: three), six received one cycle of 177Lu- satoreotide tetraxetan and 14 received two cycles. Hematologic toxicity after cycle 1 was mild-moderate and reversed before cycle 2. However, grade 4 hematologic toxicity occurred in four of seven (57%) patients after cycle 2 of 177Lu-satoreotide tetraxetan. The study was suspended, and the protocol modified to limit the cumulative absorbed bone marrow dose to 1 Gy and to reduce prescribed activity for cycle 2 by 50%. The best overall response rate was 45% [5% complete response (1/20), 40% partial response (8/20)]; with 40% stable disease (8/20) and 15% progression of disease (3/20). Median progression-free survival (PFS) was 21.0 months (95% CI, 13.6-NR).
Conclusions: In this trial of heavily treated NETs, preliminary data are promising for the use of 177Lu-satoreotide tetraxetan. Additional studies are ongoing to determine optimal therapeutic dose/schedule.
©2019 American Association for Cancer Research.
Figures


References
-
- Rinke A, Muller HH, Schade-Brittinger C, et al.: Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 27:4656–63, 2009 - PubMed
-
- Caplin ME, Pavel M, Ruszniewski P: Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 371:1556–7, 2014 - PubMed
-
- Kvols LK, Moertel CG, O’Connell MJ, et al.: Treatment of the malignant carcinoid syndrome. Evaluation of a long-acting somatostatin analogue. N Engl J Med 315:663–6, 1986 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

