Effect of dietary omega-3 fatty acids on castrate-resistant prostate cancer and tumor-associated macrophages
- PMID: 31439889
- PMCID: PMC7031053
- DOI: 10.1038/s41391-019-0168-8
Effect of dietary omega-3 fatty acids on castrate-resistant prostate cancer and tumor-associated macrophages
Abstract
Background: M2-like macrophages are associated with the pathogenesis of castrate-resistant prostate cancer (CRPC). We sought to determine if dietary omega-3 fatty acids (ω-3 FAs) delay the development and progression of CRPC and inhibit tumor-associated M2-like macrophages.
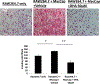
Methods: MycCap cells were grown subcutaneously in immunocompetent FVB mice. Mice were castrated when tumors reached 300 mm2. To study effects of dietary ω-3 FAs on development of CRPC, ω-3 or ω-6 diets were started 2 days after castration and mice sacrificed after early regrowth of tumors. To study ω-3 FA effects on progression of CRPC, tumors were allowed to regrow after castration before starting the diets. M2 (CD206+) macrophages were isolated from allografts to examine ω-3 FA effects on macrophage function. Omega-3 fatty acid effects on androgen-deprived RAW264.7 M2 macrophages were studied by RT-qPCR and a migration/ invasion assay.
Results: The ω-3 diet combined with castration lead to greater MycCap tumor regression (tumor volume reduction: 182.2 ± 33.6 mm3) than the ω-6 diet (tumor volume reduction: 148.3 ± 35.2; p = 0.003) and significantly delayed the time to CRPC (p = 0.006). Likewise, the ω-3 diet significantly delayed progression of established castrate-resistant MycCaP tumors (p = 0.003). The ω-3 diet (as compared to the ω-6 diet) significantly reduced tumor-associated M2-like macrophage expression of CSF-1R in the CRPC development model, and matrix metallopeptidase-9 (MMP-9) and vascular endothelial growth factor (VEGF) in the CRPC progression model. Migration of androgen-depleted RAW264.7 M2 macrophages towards MycCaP cells was reversed by addition of docosahexaenoic acid (ω-3).
Conclusions: Dietary omega-3 FAs (as compared to omega-6 FAs) decreased the development and progression of CRPC in an immunocompetent mouse model, and had inhibitory effects on M2-like macrophage function. Clinical trials are warranted evaluating if a fish oil-based diet can delay the time to castration resistance in men on androgen deprivation therapy, whereas further preclinical studies are warranted evaluating fish oil for more advanced CRPC.
Conflict of interest statement
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed by any authors.
Figures

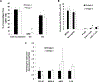

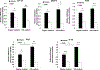

References
-
- Augustsson K, Michaud DS, Rimm EB, Leitzmann MF, Stampfer MJ, Willett WC et al. A prospective study of intake of fish and marine fatty acids and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2003; 12(1):64–67. - PubMed
-
- Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz GC, Willett WC et al. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004; 80(1):204–216. - PubMed
-
- Lovegrove C, Ahmed K, Challacombe B, Khan MS, Popert R, Dasgupta P. Systematic review of prostate cancer risk and association with consumption of fish and fish-oils: analysis of 495,321 participants. Int J Clin Pract. 2015; 69(1):87–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

