Blo t 2: Group 2 allergen from the dust mite Blomia tropicalis
- PMID: 31439916
- PMCID: PMC6706440
- DOI: 10.1038/s41598-019-48688-y
Blo t 2: Group 2 allergen from the dust mite Blomia tropicalis
Abstract
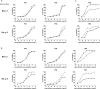
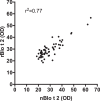
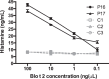
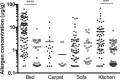
Blomia tropicalis has been recognized as a cause of allergic diseases in the tropical and subtropical regions. Here we report the immuno-characterization of its group 2 allergen, Blo t 2. Allergen Blo t 2 was amplified from the cDNA of B. tropicalis using degenerate primers, expressed in Escherichia coli as a recombinant protein and purified to homogeneity. The mature protein of Blo t 2 was 126 amino acids long with 52% sequence identity to Der p 2 and apparent molecular mass of 15 kDa. Circular dichroism spectroscopy showed that Blo t 2 is mainly a beta-sheeted protein. We confirmed the presence of three disulfide bonds in recombinant (r) Blo t 2 protein using electrospray mass spectrometry. Thirty-four percent of dust-mite allergic individuals from the Singapore showed specific IgE binding to rBlo t 2 as tested using immuno dot-blots. IgE-cross reactivity assays showed that Blo t 2 had between 20-50% of unique IgE-epitopes compared to Der p 2. IgE binding of native and recombinant forms of Blo t 2 were highly concordant (r2 = 0.77, p < 0.0001) to rBlo t 2. Dose-dependent in vitro histamine was observed when rBlo t 2 was incubated with whole blood of Blo t 2 sensitized individuals, demonstrating that it is a functional allergen. Nine naturally occurring isoforms of Blo t 2 were identified in this study, each having between 1-3 amino acid variations compared to the reference clone. Blo t 2 is a clinically relevant allergen of B. tropicalis as it has unique IgE epitopes compared to major group 2 allergens from Dermatophagoides spp.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
NMR structure and IgE epitopes of Blo t 21, a major dust mite allergen from Blomia tropicalis.J Biol Chem. 2012 Oct 5;287(41):34776-85. doi: 10.1074/jbc.M112.348730. Epub 2012 Aug 10. J Biol Chem. 2012. PMID: 22887997 Free PMC article.
-
The Blomia tropicalis allergen Blo t 7 stimulates innate immune signalling pathways through TLR2.Clin Exp Allergy. 2018 Apr;48(4):464-474. doi: 10.1111/cea.13098. Epub 2018 Feb 22. Clin Exp Allergy. 2018. PMID: 29356186
-
Cross-Reactivity between Major IgE Epitopes of Family 5 Allergens from Dermatophagoides pteronyssinus and Blomia tropicalis.Int Arch Allergy Immunol. 2019;178(1):10-18. doi: 10.1159/000492871. Epub 2018 Oct 31. Int Arch Allergy Immunol. 2019. PMID: 30380546
-
Blomia tropicalis: A 50-Year History.J Allergy Clin Immunol Pract. 2025 Jun;13(6):1289-1297. doi: 10.1016/j.jaip.2024.11.007. Epub 2024 Nov 20. J Allergy Clin Immunol Pract. 2025. PMID: 39577660 Review.
-
Molecular cloning of Blomia tropicalis allergens--a major source of dust mite allergens in the tropics and subtropics.Inflamm Allergy Drug Targets. 2006 Dec;5(4):261-6. doi: 10.2174/187152806779010954. Inflamm Allergy Drug Targets. 2006. PMID: 17168798 Review.
Cited by
-
A systematic review of allergen cross-reactivity: Translating basic concepts into clinical relevance.J Allergy Clin Immunol Glob. 2024 Feb 19;3(2):100230. doi: 10.1016/j.jacig.2024.100230. eCollection 2024 May. J Allergy Clin Immunol Glob. 2024. PMID: 38524786 Free PMC article. Review.
-
The Allergenic Activity of Blo t 2, a Blomia tropicalis IgE-Binding Molecule.Int J Mol Sci. 2023 Mar 14;24(6):5543. doi: 10.3390/ijms24065543. Int J Mol Sci. 2023. PMID: 36982614 Free PMC article.
-
Storage Mite Precision Allergy Molecular Diagnosis in the Moderate-to-Severe T2-High Asthma Phenotype.Int J Mol Sci. 2022 Apr 13;23(8):4297. doi: 10.3390/ijms23084297. Int J Mol Sci. 2022. PMID: 35457116 Free PMC article.
-
Purification and characterisation of the dimeric group 12 allergen from Blomia tropicalis heterologously expressed by Escherichia coli Top10F´.Mol Biol Rep. 2021 Apr;48(4):3405-3416. doi: 10.1007/s11033-021-06361-6. Epub 2021 Apr 29. Mol Biol Rep. 2021. PMID: 33914278
-
Frequent IgE recognition of Blomia tropicalis allergen molecules in asthmatic children and young adults in equatorial Africa.Front Immunol. 2023 Jun 2;14:1133935. doi: 10.3389/fimmu.2023.1133935. eCollection 2023. Front Immunol. 2023. PMID: 37359512 Free PMC article.
References
-
- Alimuddin S, Rengganis I, Rumende CM, Setiati S. Comparison of Specific Immunoglobulin E with the Skin Prick Test in the Diagnosis of House Dust Mites and Cockroach Sensitization in Patients with Asthma and/or Allergic Rhinitis. Acta Med Indones. 2018;50:125–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

