Dynamic CD138 surface expression regulates switch between myeloma growth and dissemination
- PMID: 31439945
- PMCID: PMC6923614
- DOI: 10.1038/s41375-019-0519-4
Dynamic CD138 surface expression regulates switch between myeloma growth and dissemination
Abstract
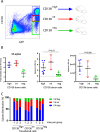
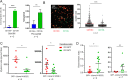
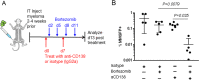
The canonical plasma cell marker CD138 (syndecan-1) is highly expressed on the myeloma cell surface, but its functional role in vivo is unclear, as well as the ontogeny of CD138-high and CD138-negative (neg) myeloma cells. In this study we used an in vivo murine Vk*MYC myeloma model where CD138 is heterogeneously expressed depending on tumor size. We find that in comparison to CD138-neg myeloma cells, the CD138-high subset of myeloma cells is highly proliferative, less apoptotic, and enhanced IL-6R signaling, which is known to promote survival. In addition CD138-high myeloma engrafts better than its CD138-neg counterpart. In contrast, CD138-neg cells are more motile both in vitro and in vivo, and more readily disseminate and spread to other bones in vivo than CD138-high subset. Neutralizing CD138 rapidly triggers migration of myeloma cells in vivo and leads to intravasation, which results in increased dissemination to other bones. Both murine and human myeloma cells can rapidly recycle CD138 surface expression through endocytic trafficking, in response to serum levels. Blocking CD138 enhances myeloma sensitivity to bortezomib chemotherapy and significantly reduces tumor size compared to bortezomib treatment alone. Thus, our data show that CD138 surface expression dynamically regulates a switch between growth vs. dissemination for myeloma, in response to nutrient conditions.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

