Synthesis of boron dipyrromethene fluorescent probes for bioorthogonal labeling
- PMID: 31439966
- PMCID: PMC6705144
- DOI: 10.1016/j.tetlet.2007.12.052
Synthesis of boron dipyrromethene fluorescent probes for bioorthogonal labeling
Abstract
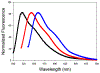
Seven 5-substituted 3-hydrazinyl derivatives of 3a, 4a-diaza-4,4-difluoro-8-phenyl boron dipyrromethene (BODIPY) were prepared for use as bioorthogonal fluorescent labels of aldehydes and ketones. The absorption energies can be tuned to absorb visible light over a large span of wavelengths by changing the nature of the 5-substituent. Optical properties of the hydrazones formed with these molecules are affected by the nature of the electrophile such that aliphatic and aromatic hydrazones can be differentiated from each other and from unreacted fluorophore.
Figures



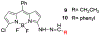
Similar articles
-
Synthesis and spectroscopic characterization of fluorescent boron dipyrromethene-derived hydrazones.J Fluoresc. 2011 Jan;21(1):347-54. doi: 10.1007/s10895-010-0723-0. Epub 2010 Oct 1. J Fluoresc. 2011. PMID: 20886269 Free PMC article.
-
8-Hydroxyquinoline-substituted boron-dipyrromethene compounds: synthesis, structure, and OFF-ON-OFF type of pH-sensing properties.J Org Chem. 2011 May 20;76(10):3774-81. doi: 10.1021/jo200050a. Epub 2011 Apr 15. J Org Chem. 2011. PMID: 21476586
-
Modulation of the spectroscopic property of Bodipy derivates through tuning the molecular configuration.Photochem Photobiol Sci. 2011 Jun;10(6):1030-8. doi: 10.1039/c1pp00001b. Epub 2011 Mar 8. Photochem Photobiol Sci. 2011. PMID: 21384046
-
BODIPY-based probes for the fluorescence imaging of biomolecules in living cells.Chem Soc Rev. 2015 Jul 21;44(14):4953-72. doi: 10.1039/c5cs00030k. Chem Soc Rev. 2015. PMID: 25801415 Review.
-
BODIPYs to the rescue: Potential applications in photodynamic inactivation.Eur J Med Chem. 2018 Jan 20;144:651-661. doi: 10.1016/j.ejmech.2017.12.068. Epub 2017 Dec 19. Eur J Med Chem. 2018. PMID: 29289888 Review.
Cited by
-
Synthesis and spectroscopic characterization of fluorescent boron dipyrromethene-derived hydrazones.J Fluoresc. 2011 Jan;21(1):347-54. doi: 10.1007/s10895-010-0723-0. Epub 2010 Oct 1. J Fluoresc. 2011. PMID: 20886269 Free PMC article.
-
Fluorescence quenchers for hydrazone and oxime orthogonal bioconjugation.Bioconjug Chem. 2012 Sep 19;23(9):1969-80. doi: 10.1021/bc300344b. Epub 2012 Aug 28. Bioconjug Chem. 2012. PMID: 22913527 Free PMC article.
-
Turn-on Fluorescent Biosensors for Imaging Hypoxia-like Conditions in Living Cells.J Am Chem Soc. 2022 May 11;144(18):8185-8193. doi: 10.1021/jacs.2c01197. Epub 2022 Apr 29. J Am Chem Soc. 2022. PMID: 35486830 Free PMC article.
-
Design and synthesis of a new class of membrane-permeable triazaborolopyridinium fluorescent probes.J Am Chem Soc. 2011 May 4;133(17):6780-90. doi: 10.1021/ja2005175. Epub 2011 Apr 7. J Am Chem Soc. 2011. PMID: 21473622 Free PMC article.
-
Functionalization of 3,5,8-trichlorinated BODIPY dyes.J Org Chem. 2014 Nov 7;79(21):10342-52. doi: 10.1021/jo501969z. Epub 2014 Oct 14. J Org Chem. 2014. PMID: 25268574 Free PMC article.
References
-
- Agard NJ; Baskin JM; Prescher JA; Lo A; Bertozzi CR, ACS Chem. Biol. 2006, 1, 644–648. - PubMed
-
- Zatsepin TS; Stetsenko DA; Gait MJ; Oretskaya TS, Bioconjug. Chem. 2005, 16, 471–489. - PubMed
-
- Cornish VW; Hahn KM; Schultz PG, J. Am. Chem. Soc. 1996, 118, 8150–8151.
-
- Zhang ZW; Smith BAC; Wang L; Brock A; Cho C; Schultz PG, Biochemistry 2003, 42, 6735–6746. - PubMed
-
- Sayer J; Peskin M; Jencks W, J. Am. Chem. Soc. 1973, 95, 4277–4287.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
