Remicade® (infliximab): 20 years of contributions to science and medicine
- PMID: 31440029
- PMCID: PMC6679695
- DOI: 10.2147/BTT.S207246
Remicade® (infliximab): 20 years of contributions to science and medicine
Abstract
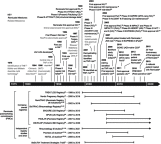
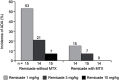
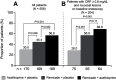
On August 24, 1998, Remicade® (infliximab), the first tumor necrosis factor-α (TNF) inhibitor, received its initial marketing approval from the US Food and Drug Administration for the treatment of Crohn's disease. Subsequently, Remicade was approved in another five adult and two pediatric indications both in the USA and across the globe. In the 20 years since this first approval, Remicade has made several important contributions to the advancement of science and medicine: 1) clinical trials with Remicade established the proof of concept that targeted therapy can be effective in immune-mediated inflammatory diseases; 2) as the first monoclonal antibody approved for use in a chronic condition, Remicade helped in identifying methods of administering large, foreign proteins repeatedly while limiting the body's immune response to them; 3) the need to establish Remicade's safety profile required developing new methods and setting new standards for postmarketing safety studies, specifically in the real-world setting, in terms of approach, size, and duration of follow-up; 4) the study of Remicade has improved our understanding of TNF's role in the immune system, as well as our understanding of the pathophysiology of a range of diseases characterized by chronic inflammation; and 5) Remicade and other TNF inhibitors have transformed treatment practices in these chronic inflammatory diseases: remission has become a realistic goal of therapy and long-term disability resulting from structural damage can be prevented. This paper reviews how, over the course of its development and 20 years of use in clinical practice, Remicade was able to make these contributions.
Keywords: Crohn’s disease; Remicade; TNF inhibition; immune-mediated inflammatory disease; infliximab; monoclonal antibody; rheumatoid arthritis.
Conflict of interest statement
RM, AG, and IA are current employees of Janssen Pharmaceuticals and stockholders in Johnson & Johnson, Janssen’s parent company. TS is a former Janssen employee and a stockholder in, and receives a pension from Johnson & Johnson. The authors report no other conflicts of interest in this work.
Figures





References
-
- REMICADE® [Package Insert]. Horsham, PA: Janssen Pharmaceuticals Inc; 2013.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

