Sex-Dependent Modulation of Acute Stress Reactivity After Early Life Stress in Mice: Relevance of Mineralocorticoid Receptor Expression
- PMID: 31440147
- PMCID: PMC6693524
- DOI: 10.3389/fnbeh.2019.00181
Sex-Dependent Modulation of Acute Stress Reactivity After Early Life Stress in Mice: Relevance of Mineralocorticoid Receptor Expression
Abstract
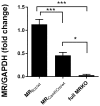
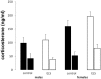
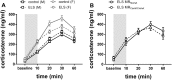
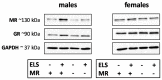
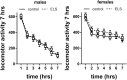
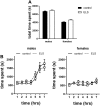
Early life stress (ELS) is considered a major risk factor for developing psychopathology. Increasing evidence points towards sex-dependent dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis as a contributing mechanism. Additionally, clinical studies suggest that the mineralocorticoid receptor (MR) may further confer genetic vulnerability/resilience on a background of ELS. The link between ELS, sex and the HPA axis and how this interacts with MR genotype is understudied, yet important to understand vulnerability/resilience to stress. We used the early life-limited nesting and bedding model to test the effect of ELS on HPA properties in adult female and male mice carrying a forebrain-specific heterozygous knockout for MR. Basal HPA axis activity was measured by circadian peak and nadir corticosterone levels, in addition to body weight and weight of stress-sensitive tissues. HPA axis reactivity was assessed by mapping corticosterone levels after 10 min immobilization. Additionally, we measured the effects of ELS on steroid receptor [MR and glucocorticoid receptor (GR)] levels in the dorsal hippocampus and medial prefrontal cortex (mPFC) with western blot. Finally, behavioral reactivity towards a novel environment was measured as a proxy for anxiety-like behavior. Results show that HPA axis activity under rest conditions was not affected by ELS. HPA axis reactivity after immobilization was decreased by ELS in females and increased, at trend-level in males. This effect in females was further exacerbated by low expression of the MR. We also observed a sex*ELS interaction regarding MR and GR expression in the dorsal hippocampus, with a significant upregulation of MR in males only. The sex-dependent interaction with ELS was not reflected in the behavioral response to novel environment and time spent in a sheltered compartment. We did find increased locomotor activity in all groups after a history of ELS, which attenuated after 4 h in males but not females regardless of condition. Our findings support that ELS alters HPA axis functioning sex-dependently. Genetic predisposition to low MR function may render females more susceptible to the harmful effect of ELS whereas in males low MR function promotes resilience. We propose that this model may be a useful tool to investigate the underlying mechanisms of sex-dependent and genetic vulnerability/resilience to stress-related psychopathology.
Keywords: HPA axis; behavior; corticosterone; early life stress (ELS); mineralocorticoid receptor; neuroendocrine; nuclear receptors; sex.
Figures

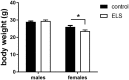
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

