The Sweet-Side of Leukocytes: Galectins as Master Regulators of Neutrophil Function
- PMID: 31440233
- PMCID: PMC6693361
- DOI: 10.3389/fimmu.2019.01762
The Sweet-Side of Leukocytes: Galectins as Master Regulators of Neutrophil Function
Abstract
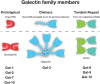
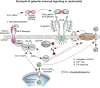
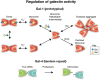
Among responders to microbial invasion, neutrophils represent one of the earliest and perhaps most important factors that contribute to initial host defense. Effective neutrophil immunity requires their rapid mobilization to the site of infection, which requires efficient extravasation, activation, chemotaxis, phagocytosis, and eventual killing of potential microbial pathogens. Following pathogen elimination, neutrophils must be eliminated to prevent additional host injury and subsequent exacerbation of the inflammatory response. Galectins, expressed in nearly every tissue and regulated by unique sensitivity to oxidative and proteolytic inactivation, appear to influence nearly every aspect of neutrophil function. In this review, we will examine the impact of galectins on neutrophils, with a particular focus on the unique biochemical traits that allow galectin family members to spatially and temporally regulate neutrophil function.
Keywords: galectin; glycans; glycoscience; inflammation; neutrophil.
Figures


References
-
- Kobayashi SD, Voyich JM, Burlak C, DeLeo FR. Neutrophils in the innate immune response. Arch Immunol Ther Exp (Warsz). (2005) 53:505–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

