AGER-Mediated Lipid Peroxidation Drives Caspase-11 Inflammasome Activation in Sepsis
- PMID: 31440260
- PMCID: PMC6694796
- DOI: 10.3389/fimmu.2019.01904
AGER-Mediated Lipid Peroxidation Drives Caspase-11 Inflammasome Activation in Sepsis
Abstract
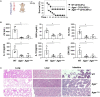
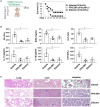
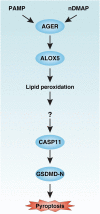
Inflammasome activation can trigger an inflammatory and innate immune response through the release of cytokines and induction of pyroptosis. A dysfunctional inflammasome has been implicated in the development of human pathologies, including sepsis and septic shock. Here, we show that advanced glycosylation end-product specific receptor (AGER/RAGE) is required for caspase-11 inflammasome activation in macrophages. A nuclear damage-associated molecular pattern (nDAMP) complex, including high-mobility group box 1, histone, and DNA, can promote caspase-11-mediated gasdermin D cleavage, interleukin 1β proteolytic maturation, and lactate dehydrogenase release. The inhibition of AGER-mediated lipid peroxidation via arachidonate 5-lipoxygenase (ALOX5) limits caspase-11 inflammasome activation and pyroptosis in macrophages in response to nDAMPs or cytosolic lipopolysaccharide. Importantly, the pharmacologic inhibition of the AGER-ALOX5 pathway or global depletion (Ager-/-) or conditional depletion of AGER in myeloid cells (AgerMye-/-) protects against lipopolysaccharide-induced septic death in poly(I:C)-primed mice. These data identify a molecular basis for caspase-11 inflammasome activation and provide a potential strategy to treat sepsis.
Keywords: AGER; ALOX5; DAMP; LPS; caspase-11; inflammasome; lipid peroxidation; sepsis.
Figures





Similar articles
-
cAMP metabolism controls caspase-11 inflammasome activation and pyroptosis in sepsis.Sci Adv. 2019 May 22;5(5):eaav5562. doi: 10.1126/sciadv.aav5562. eCollection 2019 May. Sci Adv. 2019. PMID: 31131320 Free PMC article.
-
The Protective Effects of Goitrin on LPS-Induced Septic Shock in C57BL/6J Mice via Caspase-11 Non-Canonical Inflammasome Inhibition.Molecules. 2023 Mar 23;28(7):2883. doi: 10.3390/molecules28072883. Molecules. 2023. PMID: 37049646 Free PMC article.
-
The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-Dependent Lethality in Sepsis.Immunity. 2018 Oct 16;49(4):740-753.e7. doi: 10.1016/j.immuni.2018.08.016. Epub 2018 Oct 9. Immunity. 2018. PMID: 30314759 Free PMC article.
-
Lipopolysaccharide Recognition in the Crossroads of TLR4 and Caspase-4/11 Mediated Inflammatory Pathways.Front Immunol. 2020 Nov 27;11:585146. doi: 10.3389/fimmu.2020.585146. eCollection 2020. Front Immunol. 2020. PMID: 33329561 Free PMC article. Review.
-
Caspase-11 non-canonical inflammasome: a critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses.Immunology. 2017 Oct;152(2):207-217. doi: 10.1111/imm.12787. Epub 2017 Jul 31. Immunology. 2017. PMID: 28695629 Free PMC article. Review.
Cited by
-
Emerging mechanisms of immunocoagulation in sepsis and septic shock.Trends Immunol. 2021 Jun;42(6):508-522. doi: 10.1016/j.it.2021.04.001. Epub 2021 Apr 24. Trends Immunol. 2021. PMID: 33906793 Free PMC article. Review.
-
The pathogenesis and potential therapeutic targets in sepsis.MedComm (2020). 2023 Nov 20;4(6):e418. doi: 10.1002/mco2.418. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 38020710 Free PMC article. Review.
-
A Lipid Perspective on Regulated Pyroptosis.Int J Biol Sci. 2023 Apr 25;19(8):2333-2348. doi: 10.7150/ijbs.81017. eCollection 2023. Int J Biol Sci. 2023. PMID: 37215994 Free PMC article. Review.
-
Cardiolipin inhibits the non-canonical inflammasome by preventing LPS binding to caspase-4/11.EMBO J. 2025 Aug;44(16):4419-4442. doi: 10.1038/s44318-025-00507-z. Epub 2025 Jul 16. EMBO J. 2025. PMID: 40670771 Free PMC article.
-
Redox Epiphospholipidome in Programmed Cell Death Signaling: Catalytic Mechanisms and Regulation.Front Endocrinol (Lausanne). 2021 Feb 19;11:628079. doi: 10.3389/fendo.2020.628079. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33679610 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

