Glial S100A6 Degrades β-amyloid Aggregation through Targeting Competition with Zinc Ions
- PMID: 31440382
- PMCID: PMC6675528
- DOI: 10.14336/AD.2018.0912
Glial S100A6 Degrades β-amyloid Aggregation through Targeting Competition with Zinc Ions
Abstract
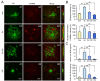
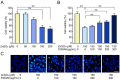
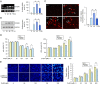
Evidence has been accumulating that zinc ions can trigger β-amyloid (Aβ) deposition and senile plaque formation in the brain, a pathological hallmark of Alzheimer's disease (AD). Chelating zinc inhibits Aβ aggregation and may hold promise as a therapeutic strategy for AD. S100A6 is an acidic Ca2+/Zn2+-binding protein found only in a small number of astrocytes in the normal brain. However, in the AD brain, S100A6 is highly expressed in astrocytes around Aβ plaques. The role of the astrocytic S100A6 upregulation in AD is unknown. In the present study, we examined the effects of S100A6 on Aβ plaques and intracellular zinc levels in a mouse model of AD. Chronic exposure to zinc increased Aβ deposition and S100A6 expression, both reversible by the zinc chelator clioquinol, in the brains of amyloid precursor protein/presenilin 1 (APP/PS1) transgenic mice. To examine whether exogenous S100A6 could induce Aβ plaque disaggregation through competition for zinc in vitro, we incubated APP/PS1 mouse brain sections with recombinant human S100A6 protein or co-incubated them with human S100A6-expressing cells. Both treatments efficiently reduced the Aβ plaque burden in situ. In addition, treatment with exogenous S100A6 protected cultured COS-7 cells against zinc toxicity. Our results show for the first time that increased S100A6 levels correlate with both Aβ disaggregation and decrease of Aβ plaque-associated zinc contents in brain sections with AD-like pathology. Astrocytic S100A6 in AD may protect from Aβ deposition through zinc sequestration.
Keywords: Alzheimer’s disease; S100A6; astrocyte; zinc; β-amyloid protein.
Conflict of interest statement
Conflict of Interest We have no conflicting interest to disclose.
Figures



Similar articles
-
Astrocytic calcium/zinc binding protein S100A6 over expression in Alzheimer's disease and in PS1/APP transgenic mice models.Biochim Biophys Acta. 2004 Dec 6;1742(1-3):161-8. doi: 10.1016/j.bbamcr.2004.09.011. Biochim Biophys Acta. 2004. PMID: 15590066
-
Clioquinol reduces zinc accumulation in neuritic plaques and inhibits the amyloidogenic pathway in AβPP/PS1 transgenic mouse brain.J Alzheimers Dis. 2012;29(3):549-59. doi: 10.3233/JAD-2011-111874. J Alzheimers Dis. 2012. PMID: 22269164
-
ApoA-I deficiency increases cortical amyloid deposition, cerebral amyloid angiopathy, cortical and hippocampal astrogliosis, and amyloid-associated astrocyte reactivity in APP/PS1 mice.Alzheimers Res Ther. 2019 May 13;11(1):44. doi: 10.1186/s13195-019-0497-9. Alzheimers Res Ther. 2019. PMID: 31084613 Free PMC article.
-
[Involvement of beta-amyloid in the etiology of Alzheimer's disease].Brain Nerve. 2010 Jul;62(7):691-9. Brain Nerve. 2010. PMID: 20675873 Review. Japanese.
-
Current understanding of metal-dependent amyloid-β aggregation and toxicity.RSC Chem Biol. 2022 Nov 22;4(2):121-131. doi: 10.1039/d2cb00208f. eCollection 2023 Feb 8. RSC Chem Biol. 2022. PMID: 36794021 Free PMC article. Review.
Cited by
-
Multifunctional Role of S100 Protein Family in the Immune System: An Update.Cells. 2022 Jul 23;11(15):2274. doi: 10.3390/cells11152274. Cells. 2022. PMID: 35892571 Free PMC article. Review.
-
Advancing Renal Amyloidosis Care: The Role of Modern Diagnostic Techniques with the Potential of Enhancing Patient Outcomes.Int J Mol Sci. 2024 May 28;25(11):5875. doi: 10.3390/ijms25115875. Int J Mol Sci. 2024. PMID: 38892061 Free PMC article. Review.
-
Zinc and Central Nervous System Disorders.Nutrients. 2023 Apr 29;15(9):2140. doi: 10.3390/nu15092140. Nutrients. 2023. PMID: 37432243 Free PMC article. Review.
-
Human neural cell type-specific extracellular vesicle proteome defines disease-related molecules associated with activated astrocytes in Alzheimer's disease brain.J Extracell Vesicles. 2022 Jan;11(1):e12183. doi: 10.1002/jev2.12183. J Extracell Vesicles. 2022. PMID: 35029059 Free PMC article.
-
APOE4 drives transcriptional heterogeneity and maladaptive immunometabolic responses of astrocytes.bioRxiv [Preprint]. 2023 Feb 6:2023.02.06.527204. doi: 10.1101/2023.02.06.527204. bioRxiv. 2023. PMID: 36798317 Free PMC article. Preprint.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
