Synthesis of Dextran-Phenoxodiol and Evaluation of Its Physical Stability and Biological Activity
- PMID: 31440502
- PMCID: PMC6694440
- DOI: 10.3389/fbioe.2019.00183
Synthesis of Dextran-Phenoxodiol and Evaluation of Its Physical Stability and Biological Activity
Abstract
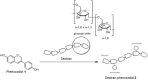
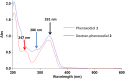
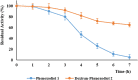
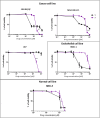
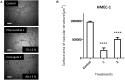
Phenoxodiol, an isoflavene anti-tumor agent, was conjugated on the polysaccharide dextran using immobilized laccase as biocatalyst. The success of the enzymatic conjugation was determined by UV-vis spectrophotometry and its functionalization degree was assessed by 1H NMR and was found to be 3.25 mg phenoxodiol/g of conjugate. An accelerated stability test showed that the resultant conjugate was nine times more stable than the free phenoxodiol when tested for its residual anti-oxidant activity with the Folin-Ciocalteu assay. The in vitro anti-proliferative activity of the conjugate was evaluated against neuroblastoma SKN-BE(2)C, triple-negative breast cancer MDA-MB-231, and glioblastoma U87 cancer cells. The conjugate was shown to be generally more potent than phenoxodiol against all three cell types tested. Additionally, the cytotoxicity and anti-angiogenic activity of the conjugate were also evaluated against non-malignant human lung fibroblast MRC-5 and human microvascular endothelial cells HMEC-1, respectively. The conjugate was found to be 1.5 times less toxic than phenoxodiol while mostly retaining 62% of its anti-angiogenic activity in the conjugate form. This study provides further evidence that the conjugation of natural product-derived drugs onto polysaccharide molecules such as dextran can lead to better stability and enhanced biological activity of the conjugate compared to the free drug alone.
Keywords: anti-angiogenic; anti-tumor; conjugate; dextran; phenoxodiol.
Figures

Similar articles
-
Synthesis of isoflavene-thiosemicarbazone hybrids and evaluation of their anti-tumor activity.Bioorg Med Chem Lett. 2017 Jun 1;27(11):2454-2458. doi: 10.1016/j.bmcl.2017.04.002. Epub 2017 Apr 3. Bioorg Med Chem Lett. 2017. PMID: 28408225
-
Preparation, characterization and in vitro biological evaluation of (1:2) phenoxodiol-β-cyclodextrin complex.Carbohydr Polym. 2017 Jun 1;165:444-454. doi: 10.1016/j.carbpol.2017.02.081. Epub 2017 Feb 22. Carbohydr Polym. 2017. PMID: 28363571
-
Synthesis, biological evaluation and structure-activity relationship studies of isoflavene based Mannich bases with potent anti-cancer activity.Bioorg Med Chem Lett. 2015 Nov 15;25(22):5377-83. doi: 10.1016/j.bmcl.2015.09.027. Epub 2015 Sep 11. Bioorg Med Chem Lett. 2015. PMID: 26432036
-
Flavonoids, phenoxodiol, and a novel agent, triphendiol, for the treatment of pancreaticobiliary cancers.Expert Opin Investig Drugs. 2009 Apr;18(4):469-79. doi: 10.1517/13543780902762835. Expert Opin Investig Drugs. 2009. PMID: 19278301 Review.
-
Phenoxodiol: pharmacology and clinical experience in cancer monotherapy and in combination with chemotherapeutic drugs.Expert Opin Pharmacother. 2009 Apr;10(6):1059-67. doi: 10.1517/14656560902837980. Expert Opin Pharmacother. 2009. PMID: 19364253 Review.
Cited by
-
Natural Ingredient-Based Polymeric Nanoparticles for Cancer Treatment.Molecules. 2020 Aug 9;25(16):3620. doi: 10.3390/molecules25163620. Molecules. 2020. PMID: 32784890 Free PMC article. Review.
-
Iron oxide nanoparticles coated with bioactive materials: a viable theragnostic strategy to improve osteosarcoma treatment.Discov Nano. 2025 Jan 30;20(1):18. doi: 10.1186/s11671-024-04163-w. Discov Nano. 2025. PMID: 39883285 Free PMC article. Review.
-
The role of PI3K/AKT signaling pathway in gallbladder carcinoma.Am J Transl Res. 2022 Jul 15;14(7):4426-4442. eCollection 2022. Am J Transl Res. 2022. PMID: 35958463 Free PMC article. Review.
-
Polysaccharide-Drug Conjugates: A Tool for Enhanced Cancer Therapy.Polymers (Basel). 2022 Feb 27;14(5):950. doi: 10.3390/polym14050950. Polymers (Basel). 2022. PMID: 35267773 Free PMC article. Review.
References
-
- Barnes S., Sfakianos J., Coward L., Kirk M. (1996). Soy isoflavonoids and cancer prevention, in Dietary Phytochemicals in Cancer Prevention and Treatment, ed American Institute for Cancer Research (Boston, MA: Springer, 87–100. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

