Stat3 in osteocytes mediates osteogenic response to loading
- PMID: 31440530
- PMCID: PMC6700265
- DOI: 10.1016/j.bonr.2019.100218
Stat3 in osteocytes mediates osteogenic response to loading
Abstract
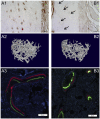
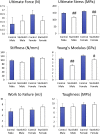
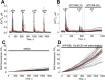
Signal transducer and activator of transcription 3 (Stat3) is a member of the Stat family of proteins involved in signaling in many different cell types, including osteocytes. Osteocytes are considered major mechanosensing cells in bone due to their intricate dendritic networks able to sense changes in physical force and to orchestrate the response of osteoclasts and osteoblasts. We examined the role of Stat3 in osteocytes by generating mice lacking Stat3 in these cells using the Dmp-1(8kb)-Cre promoter (Stat3cKO mice). Compared to age-matched littermate controls, Stat3cKO mice of either sex (18 weeks old) exhibit reduced bone formation indices, decreased osteoblasts and increased osteoclasts, and altered material properties, without detectable changes in bone mineral density (BMD) or content of either trabecular or cortical bone. In addition, Stat3cKO mice of either sex show significantly decreased load-induced bone formation. Furthermore, pharmacologic inhibition of Stat3 in osteocytes in vitro with WP1066 blocked the increase in cytosolic calcium induced by ATP, a mediator of the cellular responses to sheer stress. WP1066 also increased reactive oxygen species (ROS) production in cultured MLO-Y4 osteocytes. These data demonstrate that Stat3 is a critical mediator of mechanical signals received by osteocytes and suggest that osteocytic Stat3 is a potential therapeutic target to stimulate bone anabolism.
Keywords: ATP; Bone formation; Mechanotransduction; Osteocyte; ROS; Signal transducers and activators of transcription 3 (Stat3).
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures




References
-
- Aaronson D.S., Horvath C.M. A road map for those who don⬢t know JAK-STAT. Science. 2002;296(5573):1653–1655. - PubMed
-
- Almeida M., Han L., Martin-Millan M., O⬢Brien C.A., Manolagas S.C. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J. Biol. Chem. 2007;282(37):27298–27305. - PubMed
-
- Bakker A.D., Kulkarni R.N., Klein-Nulend J., Lems W.F. 2014. IL-6 Alters Osteocyte Signaling Toward Osteoblasts but Not Osteoclasts; pp. 394–399. - PubMed
-
- Berman A.G., Wallace J.M., Bart Z.R., Allen M.R. Raloxifene reduces skeletal fractures in an animal model of osteogenesis imperfecta. Matrix Biol. 2016;52–54:19–28. - PubMed
-
- Bivi N., Condon K.W., Allen M.R., Farlow N., Passeri G., Brun L.R., Rhee Y., Bellido T., Plotkin L.I. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2012;27(2):374–389. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

