Strategies for Phosphate Control in Patients With CKD
- PMID: 31440695
- PMCID: PMC6698320
- DOI: 10.1016/j.ekir.2019.06.002
Strategies for Phosphate Control in Patients With CKD
Abstract
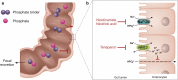
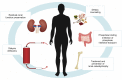
Hyperphosphatemia is a common complication in patients with chronic kidney disease (CKD), particularly in those requiring renal replacement therapy. The importance of controlling serum phosphate has long been recognized based on observational epidemiological studies that linked increased phosphate levels to adverse outcomes and higher mortality risk. Experimental data further supported the role of phosphate in the development of bone and cardiovascular diseases. Recent advances in our understanding of the mechanisms involved in phosphate homeostasis have made it clear that the serum phosphate concentration depends on a complex interplay among the kidneys, intestinal tract, and bone, and is tightly regulated by a complex endocrine system. Moreover, the source of dietary phosphate and the use of phosphate-based additives in industrialized foods are additional factors that are of particular importance in CKD. Not surprisingly, the management of hyperphosphatemia is difficult, and, despite a multifaceted approach, it remains unsuccessful in many patients. An additional issue is the fact that the supposedly beneficial effect of phosphate lowering on hard clinical outcomes in interventional trials is a matter of ongoing debate. In this review, we discuss currently available treatment approaches for controlling hyperphosphatemia, including dietary phosphate restriction, reduction of intestinal phosphate absorption, phosphate removal by dialysis, and management of renal osteodystrophy, with particular focus on practical challenges and limitations, and on potential benefits and harms.
Keywords: chronic kidney disease; dialysis; diet; hyperphosphatemia; phosphate binders; renal osteodysthrophy.
Figures
References
-
- Miyagawa A., Tatsumi S., Takahama W. The sodium phosphate cotransporter family and nicotinamide phosphoribosyltransferase contribute to the daily oscillation of plasma inorganic phosphate concentration. Kidney Int. 2018;93:1073–1085. - PubMed
-
- Isakova T., Block G. The phosphate bucket list. Kidney Int. 2018;93:1033–1035. - PubMed
-
- O'Connor L.R., Klein K.L., Bethune J.E. Hyperphosphatemia in lactic acidosis. N Engl J Med. 1977;297:707–709. - PubMed
-
- Sternbach G.L., Varon J. Severe hyperphosphatemia associated with hemorrhagic shock. Am J Emerg Med. 1992;10:331–332. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

