Neurosteroid and benzodiazepine combination therapy reduces status epilepticus and long-term effects of whole-body sarin exposure in rats
- PMID: 31440720
- PMCID: PMC6698686
- DOI: 10.1002/epi4.12344
Neurosteroid and benzodiazepine combination therapy reduces status epilepticus and long-term effects of whole-body sarin exposure in rats
Abstract
Objective: Our objective was to evaluate the protective efficacy of the neurosteroid pregnanolone (3α-hydroxy-5β pregnan-20-one), a GABAA receptor-positive allosteric modulator, as an adjunct to benzodiazepine therapy against the chemical warfare nerve agent (CWNA) sarin (GB), using whole-body exposure, an operationally relevant route of exposure to volatile GB.
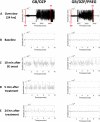
Methods: Rats implanted with telemetry transmitters for the continuous measurement of cortical electroencephalographic (EEG) activity were exposed for 60 minutes to 3.0 LCt50 of GB via whole-body exposure. At the onset of toxic signs, rats were administered an intramuscular injection of atropine sulfate (2 mg/kg) and the oxime HI-6 (93.6 mg/kg) to increase survival rate and, 30 minutes after seizure onset, treated subcutaneously with diazepam (10 mg/kg) and intravenously with pregnanolone (4 mg/kg) or vehicle. Animals were evaluated for GB-induced status epilepticus (SE), spontaneous recurrent seizures (SRS), impairment in spatial memory acquisition, and brain pathology, and treatment groups were compared.
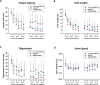
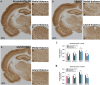
Results: Delayed dual therapy with pregnanolone and diazepam reduced time in SE in GB-exposed rats compared to those treated with delayed diazepam monotherapy. The combination therapy of pregnanolone with diazepam also prevented impairment in the Morris water maze and reduced the neuronal loss and neuronal degeneration, evaluated at one and three months after exposure.
Significance: Neurosteroid administration as an adjunct to benzodiazepine therapy offers an effective means to treat benzodiazepine-refractory SE, such as occurs following delayed treatment of GB exposure. This study is the first to present data on the efficacy of delayed pregnanolone and diazepam dual therapy in reducing seizure activity, performance deficits and brain pathology following an operationally relevant route of exposure to GB and supports the use of a neurosteroid as an adjunct to standard anticonvulsant therapy for the treatment of CWNA-induced SE.
Keywords: benzodiazepine; chemical warfare nerve agent; neurosteroids; refractory status epilepticus; thalamus.
Conflict of interest statement
Author 5 was paid under a contract to process and analyze EEG data while blinded to experimental conditions. All other authors have no conflicts of interest.
Figures




Similar articles
-
Evaluation of Midazolam-Ketamine-Allopregnanolone Combination Therapy against Cholinergic-Induced Status Epilepticus in Rats.J Pharmacol Exp Ther. 2024 Jan 17;388(2):376-385. doi: 10.1124/jpet.123.001784. J Pharmacol Exp Ther. 2024. PMID: 37770198 Free PMC article.
-
Combination of antiseizure medications phenobarbital, ketamine, and midazolam reduces soman-induced epileptogenesis and brain pathology in rats.Epilepsia Open. 2021 Dec;6(4):757-769. doi: 10.1002/epi4.12552. Epub 2021 Oct 23. Epilepsia Open. 2021. PMID: 34657398 Free PMC article.
-
Intramuscularly administered A1 adenosine receptor agonists as delayed treatment for organophosphorus nerve agent-induced Status Epilepticus.Toxicol Appl Pharmacol. 2021 May 15;419:115515. doi: 10.1016/j.taap.2021.115515. Epub 2021 Mar 30. Toxicol Appl Pharmacol. 2021. PMID: 33798593
-
Early polytherapy for benzodiazepine-refractory status epilepticus.Epilepsy Behav. 2019 Dec;101(Pt B):106367. doi: 10.1016/j.yebeh.2019.06.011. Epub 2019 Oct 18. Epilepsy Behav. 2019. PMID: 31636007 Review.
-
Neuroactive steroids for the treatment of status epilepticus.Epilepsia. 2013 Sep;54 Suppl 6(0 6):93-8. doi: 10.1111/epi.12289. Epilepsia. 2013. PMID: 24001085 Free PMC article. Review.
Cited by
-
Novel Genetically Modified Mouse Model to Assess Soman-Induced Toxicity and Medical Countermeasure Efficacy: Human Acetylcholinesterase Knock-in Serum Carboxylesterase Knockout Mice.Int J Mol Sci. 2021 Feb 14;22(4):1893. doi: 10.3390/ijms22041893. Int J Mol Sci. 2021. PMID: 33672922 Free PMC article.
-
Structure-Activity Relationship of Neuroactive Steroids, Midazolam, and Perampanel Toward Mitigating Tetramine-Triggered Activity in Murine Hippocampal Neuronal Networks.Toxicol Sci. 2021 Apr 12;180(2):325-341. doi: 10.1093/toxsci/kfab007. Toxicol Sci. 2021. PMID: 33483729 Free PMC article.
-
Protective Activity of Novel Hydrophilic Synthetic Neurosteroids on Organophosphate Status Epilepticus-induced Chronic Epileptic Seizures, Non-Convulsive Discharges, High-Frequency Oscillations, and Electrographic Ictal Biomarkers.J Pharmacol Exp Ther. 2024 Jan 17;388(2):386-398. doi: 10.1124/jpet.123.001817. J Pharmacol Exp Ther. 2024. PMID: 38050069 Free PMC article.
-
Delayed midazolam dose effects against soman in male and female plasma carboxylesterase knockout mice.Ann N Y Acad Sci. 2020 Nov;1479(1):94-107. doi: 10.1111/nyas.14311. Epub 2020 Feb 6. Ann N Y Acad Sci. 2020. PMID: 32027397 Free PMC article.
-
Long-Term Neuropsychiatric Developmental Defects after Neonatal Organophosphate Exposure: Mitigation by Synthetic Neurosteroids.J Pharmacol Exp Ther. 2024 Jan 17;388(2):451-468. doi: 10.1124/jpet.123.001763. J Pharmacol Exp Ther. 2024. PMID: 37863488 Free PMC article.
References
-
- Cannard K. The acute treatment of nerve agent exposure. J Neurol Sci 2006;249(1):86–94. - PubMed
-
- Lallement G, Dorandeu F, Filliat P, Carpentier P, Baille V, Blanchet G. Medical management of organophosphate‐induced seizures. J Physiol Paris 1998;92(5–6):369–73. - PubMed
-
- McDonough JH Jr, Shih TM. Neuropharmacological mechanisms of nerve agent‐induced seizure and neuropathology. Neurosci Biobehav Rev 1997;21(5):559–79. - PubMed
LinkOut - more resources
Full Text Sources

